Metabolic engineering of narrow-leafed lupin for the production of enantiomerically pure (-)-sparteine
Metabolic engineering of narrow-leafed lupin for the production of enantiomerically pure (-)-sparteine
Mancinotti, D.; Yang, T.; Geu-Flores, F.
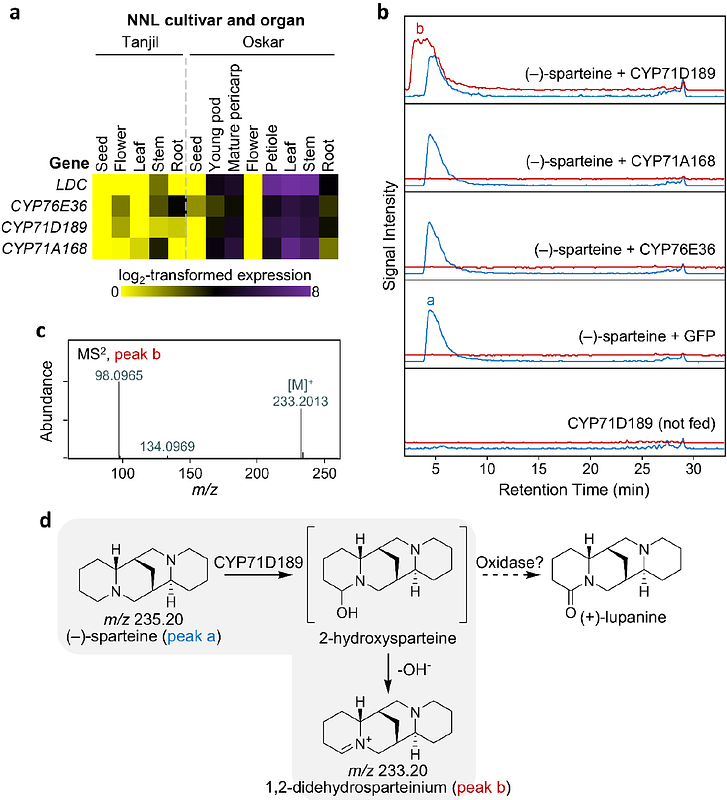
AbstractSparteine is a plant-derived alkaloid widely known for its utility as chiral ligand in asymmetric synthesis. However, its variable market price and availability have failed to meet the demand for a cheap and reliable product. Sparteine is naturally synthesized by a sub-group of legume plants, which typically accumulate complex mixtures of closely related alkaloids. Here, we identified two enzymes from narrow-leafed lupin (NLL, L. angustifolius) that can sequentially oxidize (-)-sparteine to (+)-lupanine. The first enzyme is a cytochrome P450 monooxygenase belonging to family 71 (CYP71D189) and the second one is a short-chain dehydrogenase/reductase (SDR1). We also screened a non-GMO NLL mutant library and isolated a knockout in CYP71D189. The knockout displayed an altered metabolic profile where (-)-sparteine accounted for 96% of the alkaloid content in the seeds (GC-MS basis). The (-)-sparteine isolated from the mutant seeds was enantiomerically pure (99% ee). Apart from the altered alkaloid profile, the mutant did not have any noticeable phenotype. Our work demonstrates that (-)-sparteine is the precursor of most QAs in NLL and provides a convenient source of this valuable compound for academia and industry.
