Mechanism of activation of an ancestral Tec kinase by PIP3
Mechanism of activation of an ancestral Tec kinase by PIP3
Krötenheerdt, E.; von Raussendorf, F.; Reinhardt, R.; Piëch, L.; Wedige, N.; Nyvall, H. G.; Burke, J. E.; Leonard, T. A.
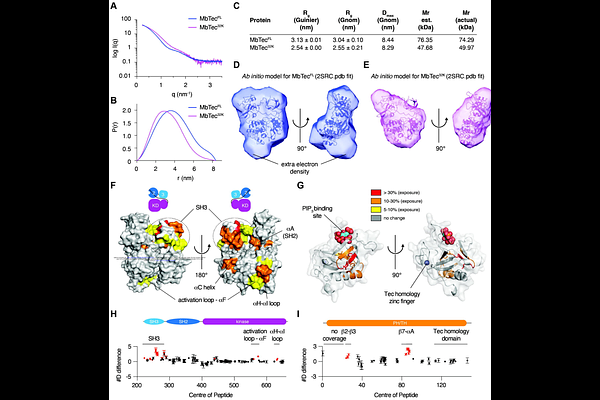
AbstractThe Tec kinases are a family of five paralogous mammalian genes that play crucial roles in cell growth, proliferation and differentiation, particularly in immune cells. The recruitment and activation of the Tec kinases depends on the generation of the lipid second messenger, PIP3, in the plasma membrane. However, the mechanisms by which PIP3 activates the Tec kinases are not well understood. In order to elucidate the mechanism by which all Tec kinases are regulated by PIP3, we studied an ancestral Tec kinase from the choanoflagellate Monosiga brevicollis. Here, we demonstrate that PIP3 relieves autoinhibition of MbTec by displacing its PH domain from an inhibitory interaction with its kinase domain. Unexpectedly, we found that a conserved polyproline motif within MbTec promotes its activation in a kinase-intrinsic mechanism. Finally, we show that the PH domain is sufficient to restore autoinhibition in a constitutively active mutant of MbTec. Our findings reveal that PIP3 is necessary, but not sufficient for MbTec activation and that the coincident detection of multiple membrane-localized signals is required to switch MbTec on.