Identification of RNF114 as ADPr-Ub reader through non-hydrolysable ubiquitinated ADP-ribose
Identification of RNF114 as ADPr-Ub reader through non-hydrolysable ubiquitinated ADP-ribose
Kloet, M.; Chatrin, C.; Mukhopadhyay, R.; van Tol, B.; Smith, R.; Rotman, S.; Tjokrodirijo, R. T. N.; Zhu, K.; Gorelik, A.; van Veelen, P. A.; Ahel, D.; Ahel, I.; van der Heden van Noort, G. J.
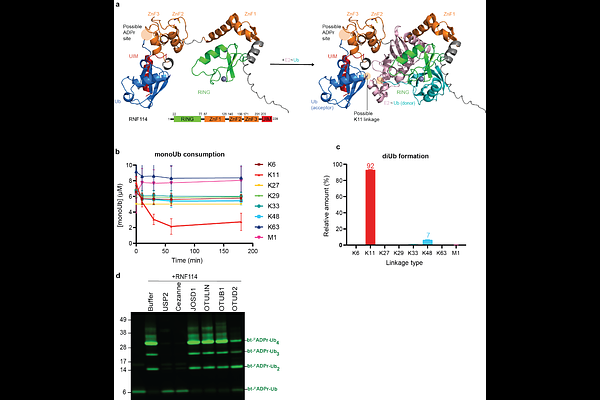
AbstractCrosstalk between the post-translational modification processes ubiquitination and ADP-ribosylation occurs in DNA-damage and immune-responses, in addition the physical linkage of ADP-ribose and ubiquitin is found during bacterial infection. Here, we study the ubiquitination of ADP-ribose mediated by human Deltex E3 ligases and the subsequent fate of the formed hybrid post-translational modification. We prepare a non-hydrolysable ADPr-Ub probe that we employ in a chemoproteomics approach and identify RNF114 as an interacting protein. Using biophysical and biochemical experiments, we validate that RNF114 preferentially interacts with ubiquitinated ADP-ribose over non-modified ubiquitin. Subsequently, RNF114 can elongate the ubiquitinated ADP-ribose with a K11-linked ubiquitin chain. Using domain deletion analysis, we pinpoint the tandem zinc fingers and ubiquitin interacting motif (ZnF2+ZnF3+UIM) domains of RNF114 to be crucial for recognising ubiquitinated ADP-ribose. Moreover, these domains are essential for the recruitment of RNF114 to the sites of laser-induced DNA damage.