CtBP1 coordinates synaptic, metabolic and contractile changes induced by denervation in skeletal muscle
CtBP1 coordinates synaptic, metabolic and contractile changes induced by denervation in skeletal muscle
Cattaneo, O.; Lopez, G.; Rajendran, J.; Chabry, F.; Prola, A.; Liaudet, N.; Startchik, S.; Castets, P.
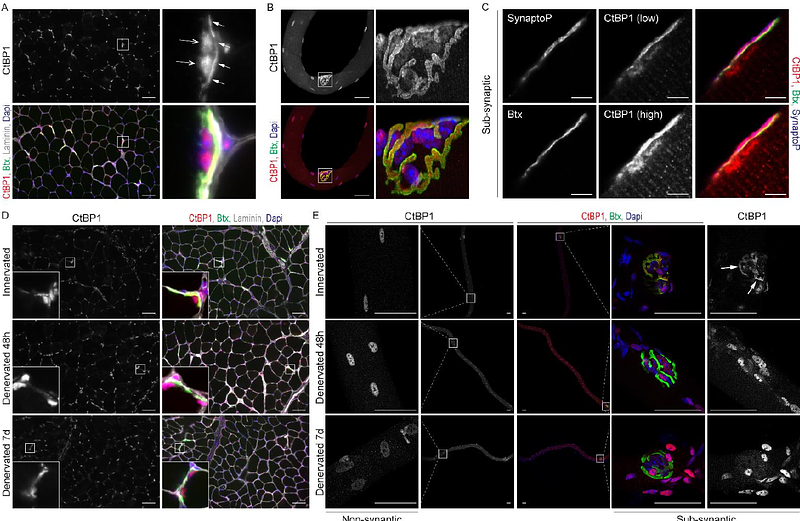
AbstractNerve injury triggers dramatic atrophy of skeletal muscle, accompanied with synaptic and metabolic changes. Regulation of denervation-induced muscle fiber remodeling involves several factors governing genetic reprogramming and proteostasis changes. Here, we demonstrate that the transcriptional co-repressor CtBP1 coordinates synaptic and metabolic changes in muscle fibers upon denervation. CtBP1 was present both in sub- and non-synaptic myonuclei in innervated muscle. Although CtBP1 levels remained unchanged in denervated muscle, CtBP1 accumulated transiently in myonuclei after 2 days of denervation. Ctbp1 knockdown perturbed the expression of a large set of activity-independent and -dependent genes in innervated and denervated skeletal muscles. CtBP1 loss had limited effect on the expression of most synaptic genes, but increased transcript levels of Chrne, encoding the adult {epsilon} sub-unit of acetylcholine receptors (AChR). However, it did not affect AChR turnover or maintenance of the post-synaptic compartment upon denervation. Importantly, we uncovered that Ctbp1 knockdown promotes denervation-induced changes in metabolic gene expression, including most genes encoding proteins of the respiratory chain complexes. Consistently, it enhanced the switch towards slower, oxidative fibers in fast muscle after 2 weeks of denervation. Moreover, CtBP1 loss precipitated the profound ultrastructural remodeling of mitochondria network induced after denervation. Hence, our study unveils the role of CtBP1 in the integrated muscle response to denervation, with important implications for CtBP1-related muscle diseases.