Allosteric modulation by the fatty acid site in the glycosylated SARS-CoV-2 spike
Allosteric modulation by the fatty acid site in the glycosylated SARS-CoV-2 spike
Oliveira, A. S. F.; Kearns, F. L.; Rosenfeld, M. A.; Casalino, L. F.; Berger, I.; Schaffitzel, C.; Davidson, A. D.; Amaro, R. E.; Mulholland, A. J.
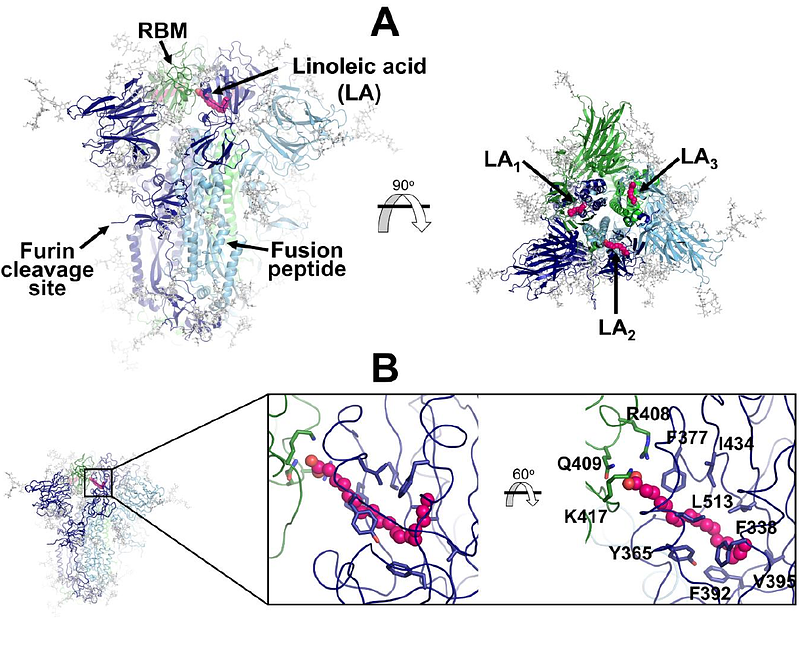
AbstractThe trimeric spike protein plays an essential role in the SARS-CoV-2 virus lifecycle, facilitating virus entry through binding to the cellular receptor angiotensin-converting enzyme 2 (ACE2) and mediating viral and host membrane fusion. The SARS-CoV-2 spike contains an allosteric fatty acid (FA) binding site at the interface between two neighbouring receptor-binding domains. This site, also found in some other coronaviruses, binds free fatty acids such as linoleic and oleic acid, and other small molecules. Understanding allostery and how this site modulates the behaviour of different regions in this protein could potentiate the development of promising alternative strategies for new coronavirus therapies. Here, we apply dynamical nonequilibrium molecular dynamics (D-NEMD) simulations to investigate allosteric effects and identify the communication pathways in the fully glycosylated spike in the original SARS-CoV-2 ancestral variant. The results reveal the allosteric networks that connect the FA site to important functional regions of the protein, including some more than 40 Angstroms away. These regions include the receptor binding motif, an antigenic supersite in the N-terminal domain, the furin cleavage site, the regions surrounding the fusion peptide and a second allosteric site known to bind heme and biliverdin. The networks identified here highlight the complexity of the allosteric modulation in this protein and reveal a striking and unexpected connection between different allosteric sites. Notably, 65% of amino acid substitutions, deletions and insertions in the Alpha, Beta, Delta, Gamma and Omicron variants map onto or close to the identified allosteric pathways.