Luminescence-based complementation assay to assess target engagement and cell permeability of glycolate oxidase (HAO1) inhibitors.
Luminescence-based complementation assay to assess target engagement and cell permeability of glycolate oxidase (HAO1) inhibitors.
Mackinnon, S. R.; Zarganes-Tzitzikas, T.; Adams, C. J.; Brennan, P. E.; Yue, W. W.
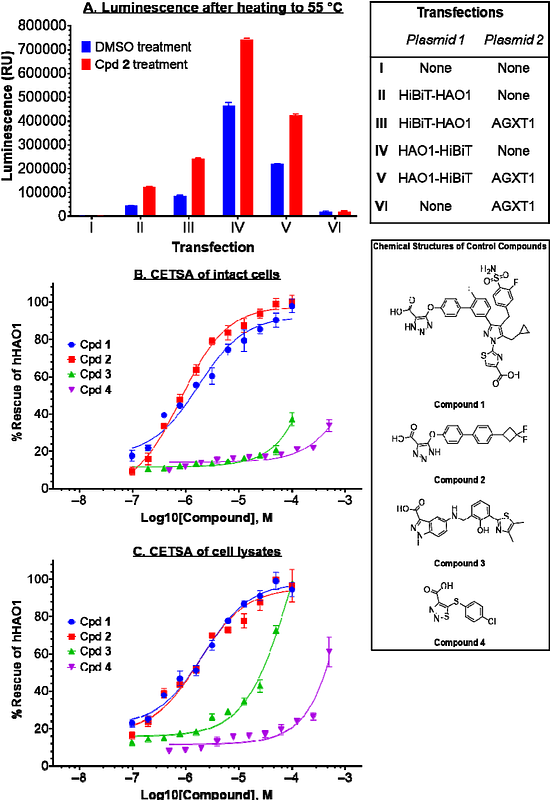
AbstractGlycolate oxidase (HAO1) catalyses the synthesis of glyoxylate, a common metabolic intermediate that causes renal failure if accumulated. HAO1 inhibition is an emerging treatment for primary hyperoxaluria, a rare disorder of glyoxylate metabolism. Here we report the first cell-based measurement of inhibitor uptake and engagement with HAO1, by adapting the cellular thermal shift assay (CETSA) based on Nano luciferase complementation and luminescence readout. By profiling the interaction between HAO1 and four well-characterised inhibitors in intact and lysed HEK293T cells, we showed that our CETSA method differentiates between low-permeability/high-engagement and high-permeability/low-engagement ligands and is able to rank HAO1 inhibitors in line with both recombinant protein methods and previously reported indirect cellular assays. Our methodology addresses the unmet need for a robust, sensitive, and scalable cellular assay to guide HAO1 inhibitor development and, in broader terms, can be rapidly adapted for other targets to simultaneously monitor compound affinity and cellular permeability.


