PFKFB2 is Pivotal for Metabolic Flexibility and Differential Glucose Utilization
PFKFB2 is Pivotal for Metabolic Flexibility and Differential Glucose Utilization
Harold, K. M.; Matsuzaki, S.; Pranay, A.; Zhu, J.; Faakye, A.; Humphries, K. M.
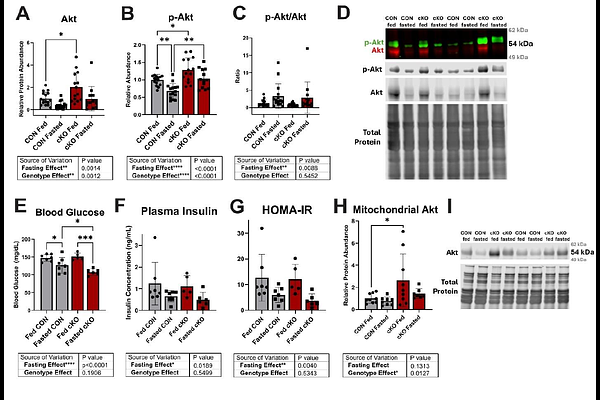
AbstractThe heart\'s constant energy demands make metabolic flexibility critical to its function as nutrient availability varies. The enzyme phosphofructokinase-2/fructose 2,6-bisphosphatase (PFKFB2) contributes to this flexibility by acting as a positive or negative regulator of cardiac glycolysis. We have previously shown that PFKFB2 is degraded in the diabetic heart and that a cardiac-specific PFKFB2 knockout (cKO) impacts ancillary glucose pathways and mitochondrial substrate preference. Therefore, defining PFKFB2\'s role in mitochondrial metabolic flexibility is paramount to understanding both metabolic homeostasis and metabolic syndromes. Further, it is unknown how PFKFB2 loss impacts the heart\'s response to acute stress. Here we examined how cardiac mitochondrial flexibility and the post-translational modification O-GlcNAcylation are affected in cKO mice in response to fasting or pharmacologic stimulation. Methods: cKO and litter-matched controls (CON) were sacrificed in the fed or fasted (12 hours) states, with or without a 20 minute stimulant stress of caffeine and epinephrine. Mitochondrial respiration, metabolomics, and changes to systemic glucose homeostasis were evaluated. Results: cKO mice had moderate impairment in mitochondrial metabolic flexibility, affecting downstream glucose oxidation, respiration, and CPT1 activity. O-GlcNAcylation, a product of ancillary glucose metabolism, was upregulated in cKO hearts in the fed state, but this was ameliorated in the fasted state. Furthermore, metabolic remodeling in response to PFKFB2 loss was sufficient to impact circulating glucose in fasted and stressed states. Conclusions: PFKFB2 is essential for fed-to-fasted changes in cardiac metabolism and plays an important regulatory role in protein O-GlcNAcylation. Its loss also affects systemic glucose homeostasis under stressed conditions.