Microtubule detyrosination alters nuclear mechanotransduction and leads to pro-hypertrophic signaling in hypertrophic cardiomyopathy
Microtubule detyrosination alters nuclear mechanotransduction and leads to pro-hypertrophic signaling in hypertrophic cardiomyopathy
Duursma, I.; Nollet, E. E.; Jansen, V. J.; Malone, J. A.; Bloem, J. S.; Bedi, K.; Margulies, K. B.; Schoonvelde, S. A. C.; Michels, M.; van der Wel, N. N.; van der Velden, J.; Kirby, T. J.; Kuster, D. W. D.
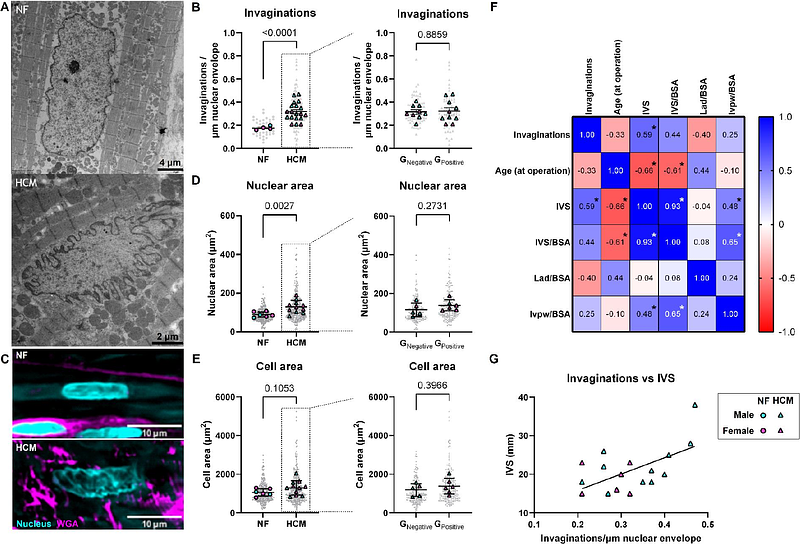
AbstractHypertrophic cardiomyopathy (HCM) is characterized by abnormal thickening of the left ventricular wall in conjunction with diastolic dysfunction. Extensive cytoskeletal remodeling takes place in HCM: increased desmin and tubulin protein levels, concomitant with an increase in stabilizing post-translational modifications such as detyrosination. We have shown that this directly contributes to diastolic dysfunction. Microtubules are connected to the nucleus by the LINC complex. Little is known about the interaction of the cytoskeleton with the nucleus and the role this may have in the development of HCM. We hypothesized that cytoskeletal remodeling increases force transmission to the nucleus, nuclear pore complex permeability and thereby pathological hypertrophy signaling. Using electron microscopy and immunofluorescent staining, we assessed nuclear morphology in cardiac septal tissue from HCM patients with obstructive HCM (N=19) compared to tissue from non-failing donors (NF, N=8). Nuclear changes were studied using wild type (WT, N=18) and homozygous Mybpc3 c.2373InsG mice (Mybpc3c.2373InsG, N=19), which have a HCM phenotype. A novel live-cell imaging approach was used to study nuclear deformation during cardiomyocyte contraction. To test functional consequences, we measured nuclear translocation of the mechanosensitive transcription factor YAP1. Nuclei were highly invaginated and enlarged in HCM in patients and the mouse model. There was a mismatch in contractile sarcomere to nuclear strain transfer indicated by restricted nuclear deformation during contraction in HCM cardiomyocytes. These findings lead to increased YAP1 nuclear translocation which induced target and pro-hypertrophic gene transcription. The nuclear abnormalities were caused by microtubule remodeling, as treatment with microtubule detyrosination inhibitor epo-Y reduced nuclear invagination, rescued sarcomere-nuclear strain transfer, and reduced YAP1 nuclear translocation. We identified extensive nuclear changes due to cytoskeletal remodeling, resulting in nuclear translocation of YAP1 and therefore altered signaling, as a driver of HCM. Targeting microtubule detyrosination rescues this phenotype implicating nuclear mechanotransduction as a potential therapeutic target for HCM.