Structural basis of liver de-targeting and neuronal tropism of CNS-targeted AAV capsids
Structural basis of liver de-targeting and neuronal tropism of CNS-targeted AAV capsids
Brittain, T. J.; Jang, S.; Coughlin, G. M.; Barcelona, B. H.; Giriat, I.; Ristic, F.; Appling, N.; Chossis, C. P.; Shay, T. F.; Gradinaru, V.
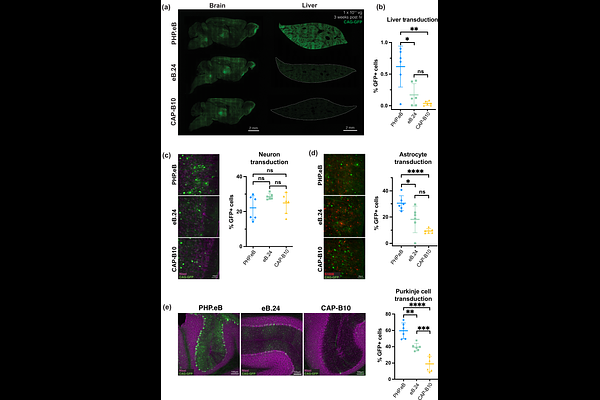
AbstractCrossing the blood-brain barrier while minimizing liver transduction is a key challenge in developing safe adeno-associated virus (AAV) vectors for treating brain disorders. In mice, the engineered capsid PHP.eB shows enhanced brain transduction, while the further engineered CAP-B10 is also de-targeted from astrocytes and liver. Here, we solve cryo-EM structures of CAP-B10 and its complex with AAV receptor (AAVR) domain PKD2, at 2.22 and 2.20 [A] resolutions, respectively. These structures reveal a structural motif that hinders AAVR binding, which we confirm by measuring affinities. We show that this motif is transferable to other capsids by solving cryo-EM structures of AAV9-X1 and AAV9-X1.1, without and with PKD2, at 3.09, 2.51, and 2.18 [A], respectively. Using this structural information, we designed and validated novel AAV variants with reduced liver and altered brain cell tropism in vivo. Overall, our findings demonstrate that rationally modulating AAVR affinity can alter liver targeting and cellular tropism.