CRISPR screen decodes SWI/SNF chromatin remodeling complex assembly
CRISPR screen decodes SWI/SNF chromatin remodeling complex assembly
Schwaemmle, H.; Soldati, H.; Lykoskoufis, N. M. R.; Docquier, M.; Hainard, A.; Braun, S. M. G.
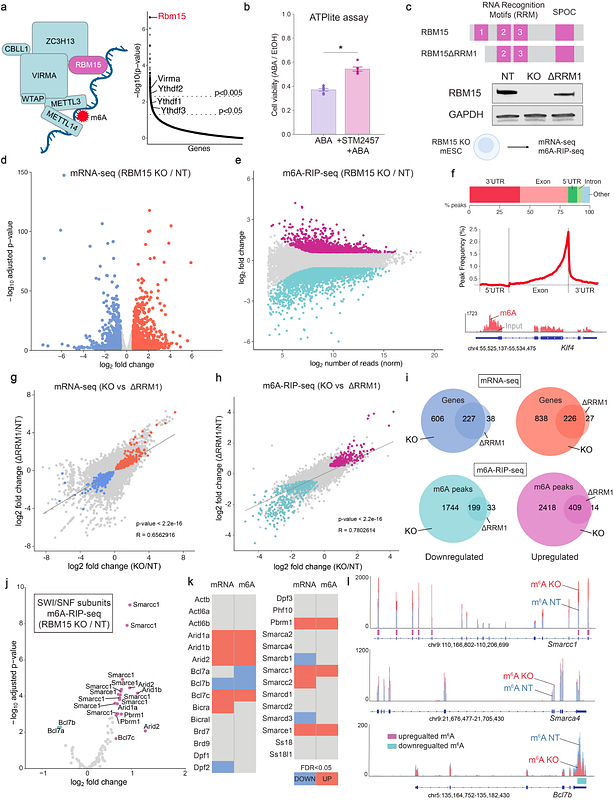
AbstractThe SWI/SNF (or BAF) complex is an essential chromatin remodeler that regulates DNA accessibility at developmental genes and enhancers. SWI/SNF subunits are among the most frequently mutated genes in cancer and neurodevelopmental disorders. These mutations are often heterozygous loss-of-function alleles, indicating a dosage-sensitive role for SWI/SNF subunits in chromatin regulation. However, the molecular mechanisms that regulate SWI/SNF subunit dosage to ensure proper complex assembly remain largely unexplored. We performed a genome-wide CRISPR KO screen, using epigenome editing in mouse embryonic stem cells, and identified Mlf2 and Rbm15 as regulators of SWI/SNF complex activity. First, we show that MLF2, a poorly characterized chaperone protein, regulates a subset of SWI/SNF target genes by promoting chromatin remodeling activity. Next, we find that RBM15, part of the m6A RNA methylation writer complex, controls m6A modifications on specific SWI/SNF mRNAs to regulate protein levels of these subunits. Misregulation of m6A methylation causes overexpression of core SWI/SNF subunits leading to the assembly of incomplete complexes lacking the catalytic ATPase/ARP subunits. These data indicate that targeting modulators of SWI/SNF complex assembly may offer a potent therapeutic strategy for diseases associated with impaired chromatin remodeling.


