Engineering a Biosynthetic Pathway for the Production of (+)-Brevianamides A and B in Escherichia coli
Engineering a Biosynthetic Pathway for the Production of (+)-Brevianamides A and B in Escherichia coli
Sandoval Hurtado, C. P.; Kelly, S. P.; Shende, V. V.; Perez, M.; Curtis, B.; Newmister, S. A.; Ott, K.; Pereira, F.; Sherman, D. H.
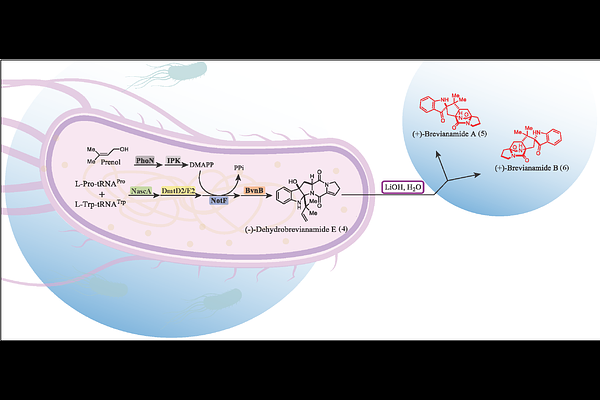
AbstractThe privileged fused-ring system comprising the bicyclo[2.2.2]diazaoctane (BDO) core is prevalent in diketopiperazine (DKP) natural products with potent and diverse biological activities, with some being explored as drug candidates. Typically, only low yields of these compounds can be extracted from native fungal producing strains and the available synthetic routes remain challenging due to their structural complexity. BDO-containing DKPs including (+)-brevianamides A and B are assembled via multi-component biosynthetic pathways incorporating non-ribosomal peptide synthetases, prenyltransferases, flavin monooxygenases, cytochrome P450s and semi-pinacolases. To simplify access to this class of alkaloids, we designed an engineered biosynthetic pathway in Escherichia coli, composed of six enzymes sourced from different kingdoms of life. The pathway includes a cyclodipeptide synthase (NascA), a cyclodipeptide oxidase (DmtD2/DmtE2), a prenyltransferase (NotF), a flavin-dependent monooxygenase (BvnB), and kinases (PhoN and IPK). Cultivated in glycerol supplemented with prenol, the engineered E. coli strain produces 5.3 mg/L of (-)-dehydrobrevianamide E (4), which undergoes a terminal, ex vivo lithium hydroxide catalyzed rearrangement reaction to yield (+)-brevianamides A and B with a 46% yield and a 92:8 diastereomeric ratio. Additionally, titers of 4 were increased eight-fold by enhancing NADPH pools in the engineered E. coli strain. Our study combines synthetic biology, biocatalysis and synthetic chemistry approaches to provide a five-step engineered biosynthetic pathway for producing complex indole alkaloids in E. coli.