Adropin protects against cardiac metabolic remodeling and dysfunction in HFpEF
Adropin protects against cardiac metabolic remodeling and dysfunction in HFpEF
Mushala, B. A.; Stoner, M. W.; Manning, J. R.; Bugga, P.; Bhattarai, N.; Sharifi-Sanjani, M.; McMahon, B.; Vandevender, A.; Mullett, S. J.; Kaufman, B. A.; Shiva, S. S.; Zhang, C.; Goetzman, E. S.; Chan, S. Y.; Gelhaus, S. L.; Jurczak, M. J.; Scott, I.
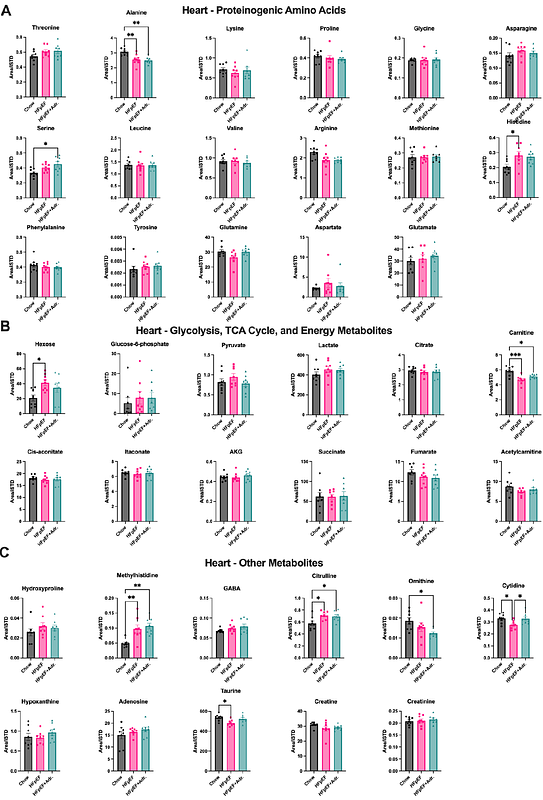
AbstractCardiometabolic heart failure with preserved ejection fraction (HFpEF) is a heterogenous metabolic disease, which in the heart presents as left ventricle diastolic dysfunction, ventricular stiffness, and myocardial structural remodeling. Deleterious changes in cardiac metabolism are central to HFpEF pathophysiology, and proposed treatments for the disease have focused on repairing these defects. In this study, we used a preclinical mouse model that recapitulates cardiometabolic HFpEF to elucidate the molecular mechanisms driving cardiac dysfunction, and tested whether recombinant Adropin (a liver- and brain-derived peptide hormone) could reverse observed defects. We show that long-term treatment with Adropin reversed multiple markers of HFpEF-related cardiac dysfunction (including fibrosis, diastolic dysfunction, and cardiomyocyte hypertrophy). Using untargeted metabolomics, we found that Adropin treatment restricted deleterious metabolite entry into the hexosamine biosynthesis pathway, leading to a reduction in the inhibitory O-GlcNAcylation of the cardiac fatty acid oxidation enzyme long chain acyl-CoA dehydrogenase. Our results suggest that Adropin may restore cardiac metabolic function in HFpEF, and that targeting this pathway may be a novel therapeutic avenue for this disease.