Intricate mechanism (s) of substrate specificity and loss of function on disease mutation (K211N) of Parkin
Intricate mechanism (s) of substrate specificity and loss of function on disease mutation (K211N) of Parkin
Lenka, D. R.; Chaurasiya, S.; Kumar, A.
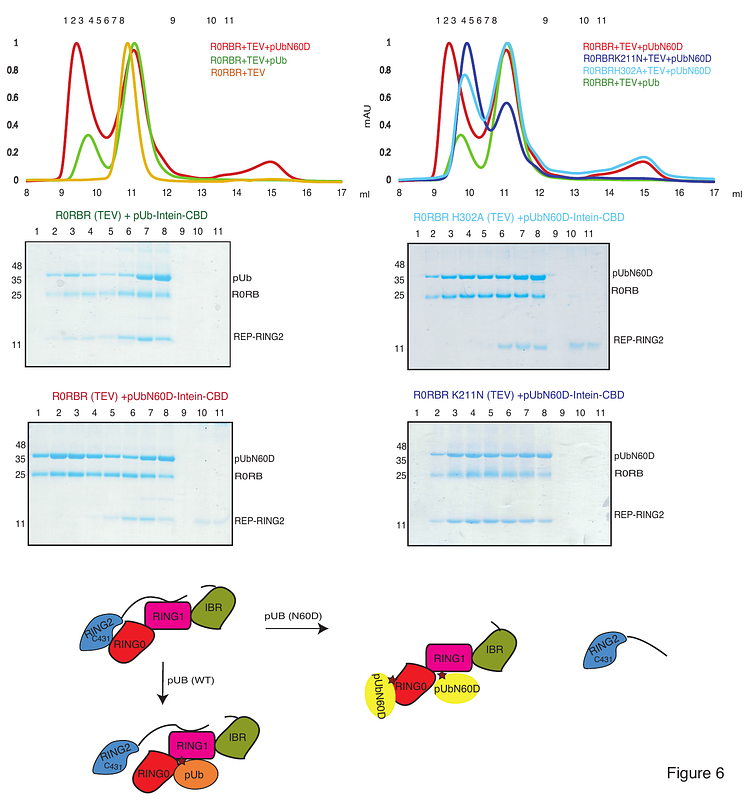
AbstractParkin mutations, including K211N, lead to the onset of Parkinson\'s disease. Ubiquitin-like proteins (NEDD8, Ubiquitin) and Ubiquitin-like (Ubl) domains of Parkin are phosphorylated by PINK1. Parkin is activated by binding of phospho-ubiquitin, phospho-NEDD8, and phospho-Ubl domain on the two distinct pockets of Parkin. While pUb/pNEDD8 binds in the RING1 pocket, pUb/pUbl binding with the RING0 pocket is reported. pUb binding in the RING0 pocket (comprising K211) is believed to rationalize the loss of pUb-mediated Parkin K211N activation. Herein, we demonstrate that the RING0 pocket is specific for pUbl and does not bind with pUb/pNEDD8. In contrast, the RING1 pocket is more suitable for binding with phospho-NEDD8 than phospho-ubiquitin. Also, we demonstrate that the binding of activators in the RING1 and RING0 pockets of Parkin leads to a distinct extent of conformational changes on the RING2. We also show that loss of Parkin activity in disease mutation K211N is independent of Parkin activators (pUb/pNEDD8) binding. Furthermore, the crystal structure of Parkin K211N in complex with pNEDD8 reveals the mechanism of Parkin inactivation due to conformational changes locking RING2 with RING0. This study would help design small-molecule Parkin activators against Parkinson\'s disease.