Mechanistic modeling identifies emergent properties of tandem SH2 domain interactions
Mechanistic modeling identifies emergent properties of tandem SH2 domain interactions
Portelance, R.; Wu, A.; Kandoor, A.; Naegle, K. M.
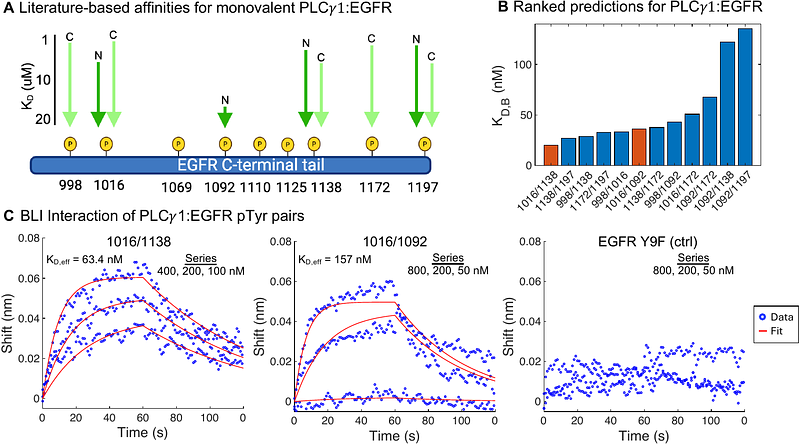
AbstractTandem SH2 domains occur in key protein mediators of phosphotyrosine signaling and have the capacity to drive high affinity interactions through the avidity that results with bisphosphorylated protein partners. However, challenges have prevented the broad exploration of tandem SH2 domain avidity and here we utilize advances in both computational modeling and experimental approaches to predict and test tandem SH2 domain recruitment. Theoretical model behavior suggests that maximum avidity occurs with closely spaced or flexibly linked phosphotyrosine sites, combined with moderate monovalent affinities - exactly around the affinities of SH2 domains with individual phosphotyrosine sites. Surprisingly, despite sequence diversity, structure-based analysis showed remarkably conserved three-dimensional spacing between SH2 domains across all tandem SH2 families, which we interrogate experimentally, suggesting evolutionary optimization for avidity interactions. The combination of structure-based analysis of domain spacing with available monovalent experimental data appears to be sufficiently accurate to rank order predict high affinity interactions of tandem SH2 domain recruitment to the EGFR C-terminal tail. These approaches lay the groundwork for larger utility in multivalent prediction and testing to help better understand protein interactions that drive cell signaling.