Substitutions at the C-8 position of quinazolin-4-ones improve the potency of nicotinamide site binding tankyrase inhibitors
Substitutions at the C-8 position of quinazolin-4-ones improve the potency of nicotinamide site binding tankyrase inhibitors
Bosetti, C.; Kampasis, D.; Galera-Prat, A.; Brinch, S. A.; Karelou, M.; Dhakar, S. S.; Alaviuhkola, J.; Waaler, J.; Lehtiö, L.; Kostakis, I.
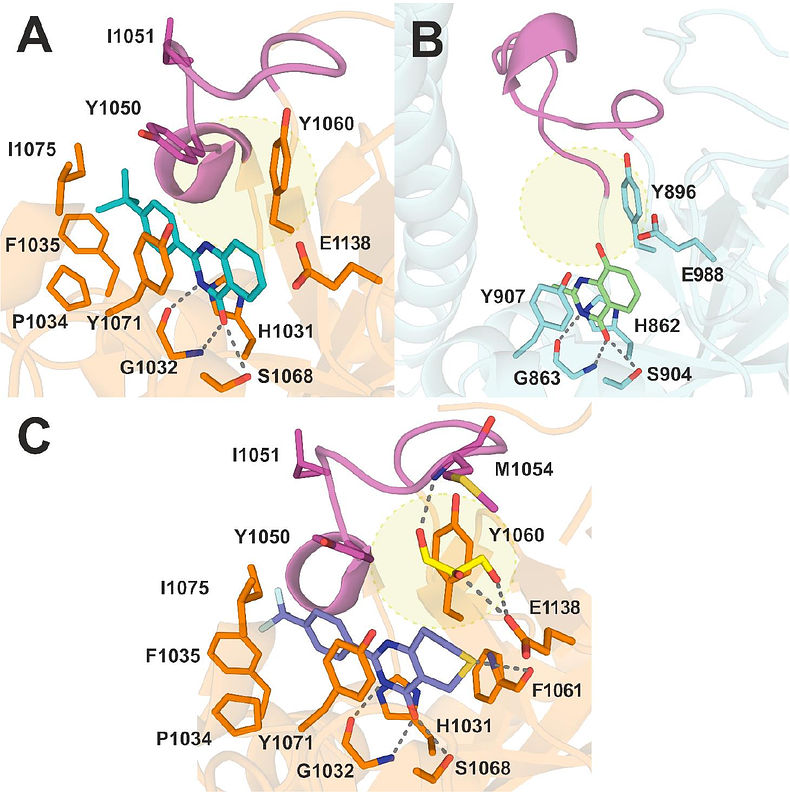
AbstractHuman diphtheria toxin-like ADP-ribosyltransferases, PARPs and tankyrases, transfer ADP-ribosyl groups to other macromolecules, thereby controlling various signaling events in cells. They are considered promising drug targets, especially in oncology, and some small molecule inhibitors have already been developed. These inhibitors typically interact with the nicotinamide binding site and extend along the NAD+ binding groove of the catalytic domain. Quinazolin-4-ones have been explored as promising scaffolds for such inhibitors and we have identified a new position within the catalytic domain that has not been extensively studied yet. In this study, we investigate larger substituents at the C-8 position and, using X-ray crystallography, we demonstrate that nitro- and diol-substituents engage in new interactions with TNKS2, improving both affinity and selectivity. Both nitro- and diol- substituents exhibit intriguing inhibition of TNKS2, with compound 49 displaying an IC50 of 65 nM, while compound 40\'s IC50 value is 14 nM. Both analogues show efficacy in cell assays and attenuate the tankyrase-controlled Wnt/{beta}-catenin signaling with sub-micromolar IC50. When tested against a wider panel of enzymes, compound 40 displayed high selectivity towards tankyrases, whereas 49 also inhibited other PARPs. The results offer new insights for inhibitor development targeting tankyrases and PARPs by focusing on the subsite between a mobile active site loop and the canonical nicotinamide binding site.


