Single-stranded DNA binding protein hitches a ride with the Escherichia coli YoaA-χ helicase
Single-stranded DNA binding protein hitches a ride with the Escherichia coli YoaA-χ helicase
Weeks-Pollenz, S. J.; Petrides, M. J.; Davis, R.; Harris, K. K.; Bloom, L.
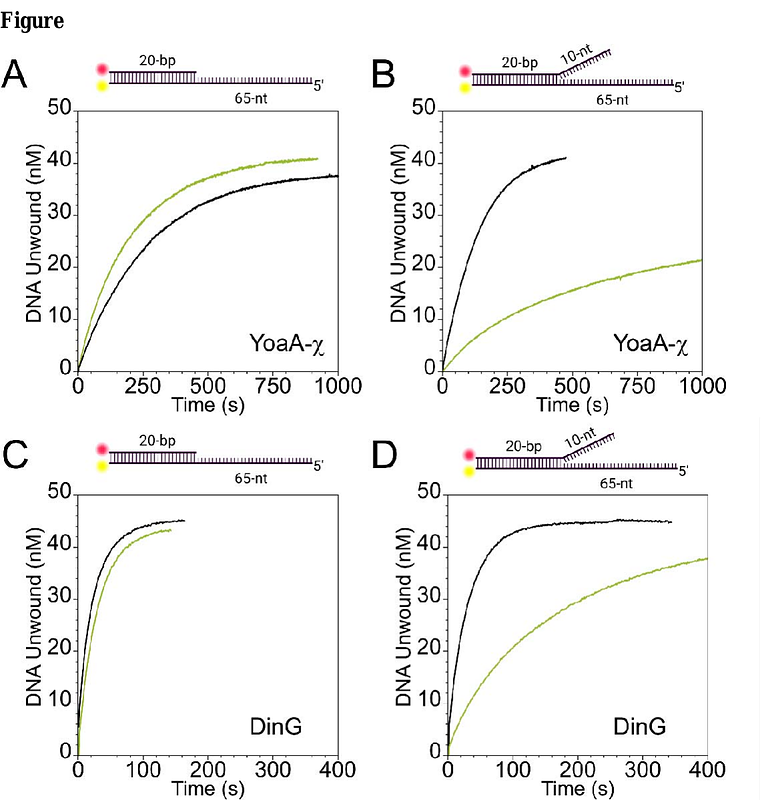
AbstractThe Escherichia coli XPD/Rad3-like helicase, YoaA, and DNA polymerase III subunit, {chi}, are involved in E. coli DNA damage tolerance and repair. YoaA and {chi} promote tolerance to the DNA chain-terminator, 3\'-azidothymidine (AZT), and together form the functional helicase complex, YoaA-{chi}. How YoaA-{chi} contributes to DNA damage tolerance is not well understood. E. coli single-stranded DNA binding protein (SSB) accumulates at stalled replication forks, and the SSB-{chi} interaction is required to promote AZT tolerance via an unknown mechanism. YoaA-{chi} and SSB interactions were investigated in vitro to better understand this DNA damage tolerance mechanism, and we discovered YoaA-{chi} and SSB have a functional interaction. SSB confers a substrate-specific effect on the helicase activity of YoaA-{chi}, barely affecting YoaA-{chi} on an overhang DNA substrate but inhibiting YoaA-{chi} on forked DNA. A paralog helicase, DinG, unwinds SSB-bound DNA in a similar manner to YoaA-{chi} on the substrates tested. Through use of ensemble experiments, we believe SSB binds behind YoaA-{chi} relative to the DNA ds/ss junction and show via single-molecule assays that SSB translocates along ssDNA with YoaA-{chi}. This is, to our knowledge, the first demonstration of a mechanoenzyme pulling SSB along ssDNA.


