Resolving Challenges in Detection and Quantification of D-2-hydroxyglutarate and L-2-hydroxyglutarate via LC/MS
Resolving Challenges in Detection and Quantification of D-2-hydroxyglutarate and L-2-hydroxyglutarate via LC/MS
Dowdy, T.; Larion, M.
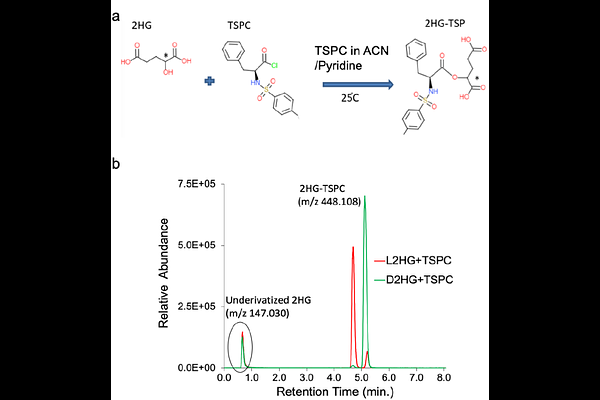
AbstractD-2-Hydroxyglutarate and L-2-Hydroxyglutarate (D-2HG/L-2HG) are typically metabolites of non-specific enzymatic reactions that are kept in check by the housekeeping enzymes, D-2HG /L-2HG dehydrogenase (D-2HGDH/L-2HGDH). In certain disease states, such as D-2HG or L-2HG aciduria and cancers, accumulation of these biomarkers interferes with oxoglutarate-dependent enzymes that regulate bioenergetic metabolism, histone methylation, post-translational modification, protein expression and others. D-2HG has a complex role in tumorigenesis that drives metabolomics investigations. Meanwhile, L-2HG is produced by non-specific action of malate dehydrogenase and lactate dehydrogenase under acidic or hypoxic environments. Characterization of divergent effects of D-2HG/L-2HG on the activity of specific enzymes in diseased metabolism depends on their accurate quantification via mass spectrometry. Despite advancements in high-resolution quadrupole time-of-flight mass spectrometry (HR-QTOF-MS), challenges are typically encountered when attempting to resolve of isobaric and isomeric metabolites such as D-2HG/L-2HG for quantitative analysis. Herein, available D-2HG/L-2HG derivatization and liquid chromatography (LC) MS quantification methods were examined. The outcome led to the development of a robust, high-throughput HR-QTOF-LC/MS approach that permits concomitant quantification of the D-2HG and L-2HG enantiomers with the benefit to quantify the dysregulation of other intermediates within interconnecting pathways. Calibration curve was obtained over the linear range of 0.8-104 nmol/mL with r2 [≥] 0.995 for each enantiomer. The LC/MS-based assay had an overall precision with intra-day CV % [≤] 8.0 and inter-day CV % [≤] 6.3 across the quality control level for commercial standard and pooled biological samples; relative error % [≤] 2.7 for accuracy; and resolution, Rs= 1.6 between 2HG enantiomers (m/z 147.030), D-2HG and L-2HG (at retention time of 5.82 min and 4.75 min, respectively) following chiral derivatization with diacetyl-L-tartaric anhydride (DATAN). Our methodology was applied to disease relevant samples to illustrate the implications of proper enantioselective quantification of both D-2HG and L-2HG. The stability of the method allows scaling to large cohorts of clinical samples in the future.