A CLIC1 network coordinates matrix stiffness and the Warburg effect to promote tumor growth in pancreatic cancer
A CLIC1 network coordinates matrix stiffness and the Warburg effect to promote tumor growth in pancreatic cancer
Zheng, J.-H.; Zhu, Y.-H.; Yang, J.; Ji, P.-X.; Zhao, R.-K.; Duan, Z.-H.; Yao, H.-F.; Jia, Q.-Y.; Yin, Y.-F.; Hu, L.-P.; Li, Q.; Jiang, S.-H.; Huo, Y.-M.; Liu, W.; Sun, Y.-W.; Liu, D.-J.
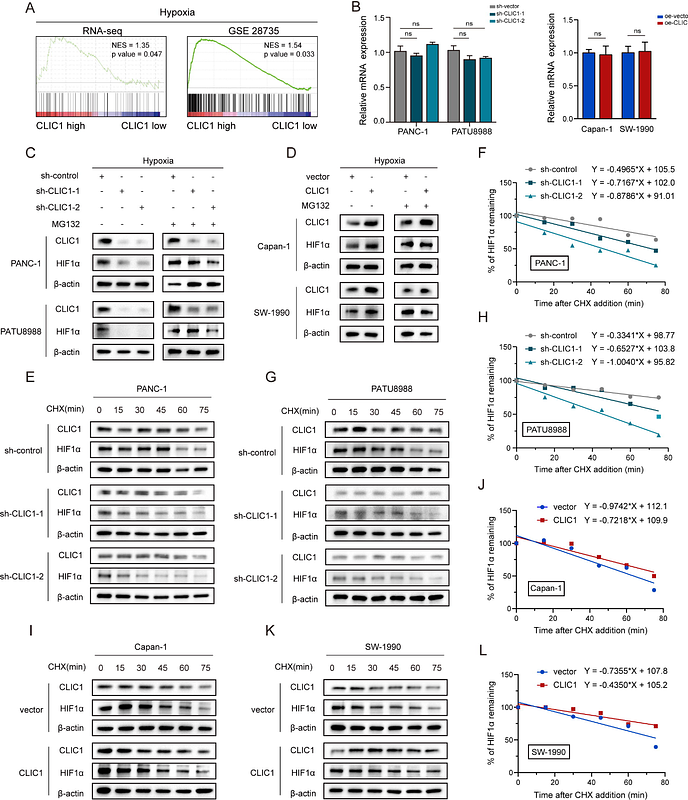
AbstractBACKGROUND & AIMS: PDAC is characterized by significant matrix stiffening and reprogrammed glucose metabolism, particularly the Warburg effect. However, it is not clear the connection between matrix stiffness and the Warburg effect and the mechanisms of action in tumor progression. METHODS: The relationship between matrix stiffness and the Warburg effect was investigated from clinical, cellular, and bioinformatical perspectives. The ChIP and luciferase reporter gene assays were used to clarify the regulation mechanism of matrix stiffness on the expression of CLIC1. The expression profile and clinical significance of CLIC1 were determined in GEO datasets and a TMA. Loss-of-function and gain-of-function technics were used to determine the in vitro and in vivo functions of CLIC1. GSEA and western blotting revealed the underlying molecular mechanisms. RESULTS: PDAC matrix stiffness is closely associated with the Warburg effect, and CLIC1 is a key molecule connecting tumor matrix stiffness and the Warburg effect. Increased CLIC1 expression induced by matrix stiffness correlates with poor prognosis in PDAC. CLIC1 acts as a promoter of glycolytic metabolism and facilitates tumor growth in a glycolysis-dependent manner. Mechanistically, CLIC1 inhibits the hydroxylation of HIF1alpha via ROS, which then increases the stability of HIF1alpha. Collectively, PDAC cells can sense extracellular matrix stiffness and upregulate the expression of CLIC1, which facilitates the Warburg effect through ROS/HIF1alpha signaling, thereby supporting tumor growth. CONCLUSIONS: In the context of tumor therapy, targeted approaches can be considered from the perspectives of both extracellular matrix stiffness and tumor metabolism, of which CLIC1 is one of the targets.