Ketone Bodies-mediated Cysteine Modifications Discovered by Chemical Proteomics
Ketone Bodies-mediated Cysteine Modifications Discovered by Chemical Proteomics
Zhou, Y.-F.; Zhang, L.; Niu, Z. L.; WANG, X.; Hunt, R.; Zhao, Y.; Sharifi, N.; Wang, Z. A.
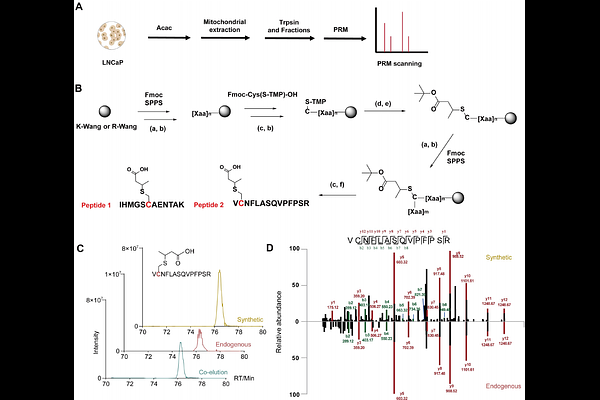
AbstractAll the studies of ketone body-dependent post-translational modifications (PTMs), notably those mediated by ketone bodies, {beta}-hydroxybutyrate (Bhb) and acetoacetate (Acac), have focused on lysine acylations. However, given the chemically diverse and reactive nature of metabolites generated, it remains unclear whether non-lysine modifications can also happen. Here, we report the synthesis of an acetoacetate-alkyne (Acac-alkyne) chemical probe that enables efficient metabolic labeling, robust fluorescent visualization, and mass spectrometry-based identification of Acac-modified proteins. By combining chemical proteomics with open-search strategy, we showed that Acac will induce previously uncharacterized cysteine modifications in mammalian cells. Notably, cysteine S-crotonation (Ccr) is validated by employing both probe-based and standard peptide-based co-elution assays. Metabolic pathway tracing further identifies BDH1 and ECHS1 as key enzymes that generate Ccr formation. Together, these findings establish ketone metabolism as a novel source of cysteine modifications and provide an alternative mechanistic pathway to explain the profound biological effects of ketone body.