Exploration of individual beta cell function over time in vivo: effects of hyperglycemia and glucagon-like peptide-1 receptor (GLP1R) agonism
Exploration of individual beta cell function over time in vivo: effects of hyperglycemia and glucagon-like peptide-1 receptor (GLP1R) agonism
Delgadillo, L.; Salazar, S.; Lopez-Noriega, L.; Provencher-Girard, A.; Larouche, S.; Prat, A.; Rutter, G. A.
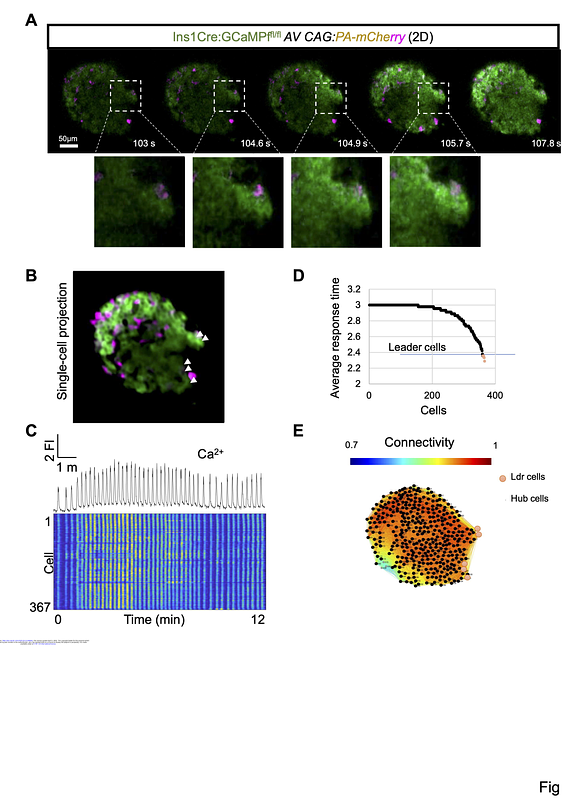
AbstractThe coordinated function of beta cells within the pancreatic islet is required for the normal regulation of insulin secretion and is partly controlled by specialized leader and highly connected hub beta-cell subpopulations. Whether cells within these subpopulations are functionally stable in vivo remains unclear. Here, we establish an approach to monitor Ca2+ dynamics within individual beta cells over time, after engraftment into the anterior eye chamber, where continuous blood perfusion and near normal innervation pertain. Under normoglycemic conditions, islet network dynamics, and the behavior of individual leaders and hubs, remain stable for at least seven days. Hyperglycemia, resulting from high-fat diet feeding or the loss of a host Gck allele, caused engrafted islets to display incomplete and abortive Ca2+ waves and overall connectivity was diminished. Whereas hub cell numbers were lowered profoundly in both disease models, leaders largely persisted. Treatment with the GLP1R agonist Exendin-4 led to a recovery of islet-wide Ca2+ dynamics and the re-emergence of hub cells within minutes, with the effects of the incretin mimetic being more marked than those observed after analogous treatments in vitro. Similar observations were made using 3-dimensional imaging across the whole islet. Our findings thus suggest that incretins may act both directly and indirectly on beta cells in vivo. The approach described may provide broad applicability to the exploration of individual cell function over time in the living animal.