Self-organized spatial targeting of contractile actomyosin rings for synthetic cell division
Self-organized spatial targeting of contractile actomyosin rings for synthetic cell division
Reverte-Lopez, M.; Kanwa, N.; Qutbuddin, Y.; Jasnin, M.; Schwille, P.
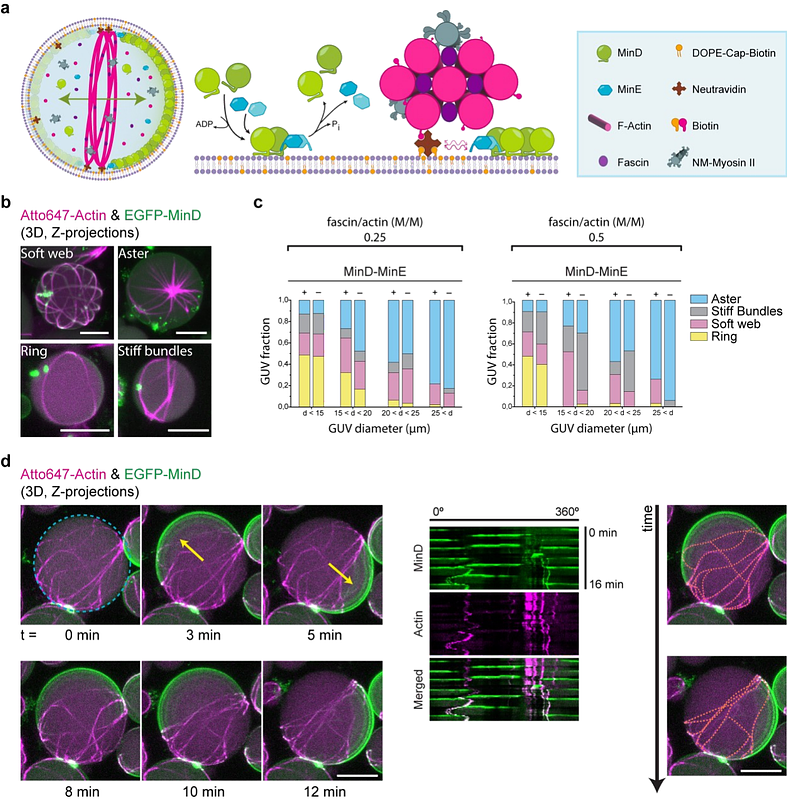
AbstractOne of the challenges of bottom-up synthetic biology is the engineering of a minimal module for self-division of synthetic cells. To produce the contractile forces required for the controlled excision of cell-like compartments such as giant unilamellar vesicles (GUVs), reconstituted cytokinetic rings made of actin are considered to be among the most promising structures of a potential synthetic division machinery. Although the targeting of actin rings to GUV membranes and their myosin-induced constriction have been previously demonstrated, large-scale vesicle deformation has been precluded due to the lacking spatial control of these contractile structures. Here, we show the combined in vitro reconstitution of actomyosin rings and the bacterial MinDE protein system, effective in targeting E.coli Z-rings to mid-cell, within GUVs. Incorporating this spatial positioning tool, which induces active transport of any diffusible molecule on membranes, yields self-organized assembly of actomyosin rings at the equatorial plane of vesicles. Remarkably, the synergistic effect of Min oscillations and the contractile nature of actomyosin bundles induces mid-vesicle membrane deformation and striking bleb-like protrusions, leading to shape remodeling and symmetry breaking. Our system showcases how functional machineries from various organisms may be synergistically combined in vitro, leading to the emergence of new functionality towards a synthetic division system.


