Design, Construction, and Validation of a Yeast-Displayed Chemically Expanded Antibody Library
Design, Construction, and Validation of a Yeast-Displayed Chemically Expanded Antibody Library
Rezhdo, A.; Hershman, R. L.; Van Deventer, J. A.
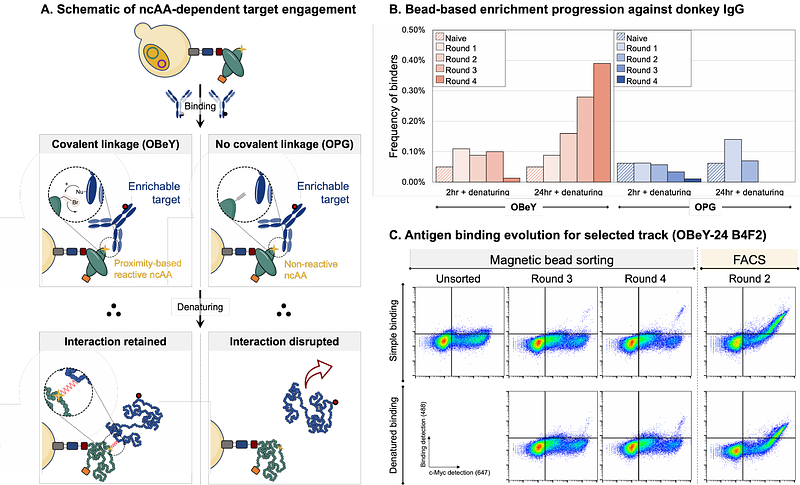
AbstractIn vitro display technologies, exemplified by phage and yeast display, have emerged as powerful platforms for antibody discovery and engineering. However, the identification of antibodies that disrupt target functions beyond binding remains a challenge. In particular, there are very few strategies that support identification and engineering of either protein-based irreversible binders or inhibitory enzyme binders. Expanding the range of chemistries in antibody libraries has the potential to lead to efficient discovery of function-disrupting antibodies. In this work, we describe a yeast display-based platform for the discovery of chemically diversified antibodies. We constructed a billion-member antibody library that supports the presentation of a range of chemistries within antibody variable domains via noncanonical amino acid (ncAA) incorporation and subsequent bioorthogonal click chemistry conjugations. Use of a polyspecific orthogonal translation system enables introduction of chemical groups with various properties, including photo-reactive, proximity-reactive, and click chemistry-enabled functional groups for library screening. We established conjugation conditions that facilitate modification of the full library, demonstrating the feasibility of sorting the full billion-member library in protein-small molecule hybrid format in future work. Here, we conducted initial library screens after introducing O-(2-bromoethyl)tyrosine (OBeY), a weakly electrophilic ncAA capable of undergoing proximity-induced crosslinking to a target. Enrichments against donkey IgG and protein tyrosine phosphatase 1B (PTP1B) each led to the identification of several OBeY-substituted clones that bind to the targets of interest. Flow cytometry analysis on the yeast surface confirmed higher retention of binding for OBeY-substituted clones compared to clones substituted with ncAAs lacking electrophilic side chains after denaturation. However, subsequent crosslinking experiments in solution with ncAA-substituted clones yielded inconclusive results, suggesting that weakly reactive OBeY side chain is not sufficient to drive robust crosslinking in the clones isolated here. Nonetheless, this work establishes a multi-modal, chemically expanded antibody library and demonstrates the feasibility of conducting discovery campaigns in chemically expanded format. This versatile platform offers new opportunities for identifying and characterizing antibodies with properties beyond what is accessible with the canonical amino acids, potentially enabling discovery of new classes of reagents, diagnostics, and even therapeutic leads.


