Mating Type Gene Divergence is Associated with Life Cycle Differentiation in Scots Pine Blister Rust
Mating Type Gene Divergence is Associated with Life Cycle Differentiation in Scots Pine Blister Rust
Gomez-Zapata, P. A.; Tellgren-Roth, C.; Samils, B.; Stenlid, J.; Kaitera, J.; Brandstrom Durling, M.; Seidl, M. F.; Olson, A.
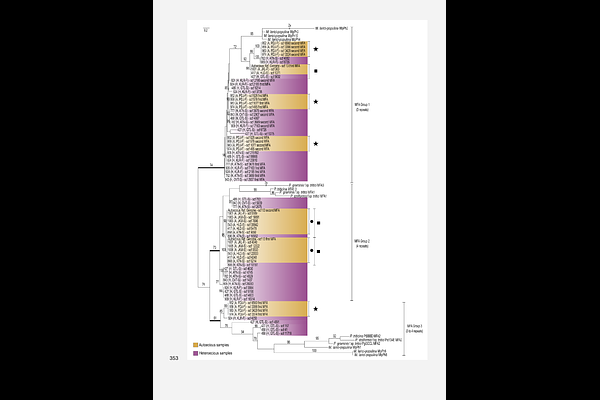
AbstractReproductive systems are central to the evolutionary and ecological processes shaping population structure and adaptability. Rust fungi are a large group of obligate plant pathogens with complex life cycles and diverse reproductive modes. In the rust fungus Cronartium pini, which exists in both macrocyclic heteroecious and microcyclic autoecious forms, transitions between reproductive modes can be studied within a single species. Here, we used comparative genomics to analyse the structure, diversity, and organization of mating-type (MAT) loci across both forms of C. pini. We identified a canonical tetrapolar system in the heteroecious form, characterized by unlinked, multiallelic homeodomain (HD) and pheromone/receptor (P/R) loci, consistent with obligate outcrossing. In contrast, the autoecious form displayed distinct reproductive signatures, including MAT gene homozygosity in some samples, indicative of clonal reproduction, and MAT gene duplications in others, suggesting self-fertility or altered mating pathways. Three HD loci were detected, but only one exhibited high allelic diversity and was consistently present, indicating functional divergence. Expanded allelic diversity at the STE3.2 pheromone receptor further suggests plasticity at the P/R locus. Although allele sharing between life cycle forms was rare, isolated cases of shared MAT alleles suggest limited historical connectivity and possible trans-specific polymorphism. Together, our findings reveal flexibility in the mating system architecture of C. pini and suggest that transitions between sexual and asexual reproduction may be facilitated by retained, functionally ambiguous MAT structures. This work highlights C. pini as a powerful model for studying life cycle evolution and mating system transitions in rust fungi.