Decreased Extracellular Vesicle Vasorin in Severe Preeclampsia Plasma Mediates Endothelial Dysfunction
Decreased Extracellular Vesicle Vasorin in Severe Preeclampsia Plasma Mediates Endothelial Dysfunction
Murugesan, S.; Addis, D. R. R.; Hussey, H.; Powell, M. F.; Saravanakumar, L.; Sturdivant, A. B.; Sinkey, R. G.; Tubinis, M. D.; Massey, Z. R.; Mobley, J. A.; Tita, A. N.; Jilling, T.; Berkowitz, D. E.
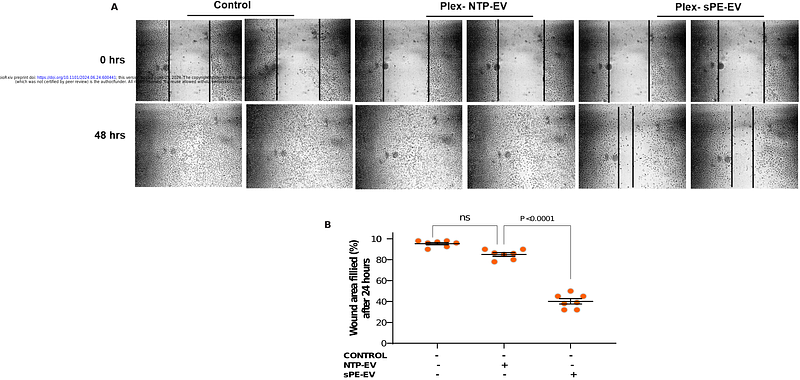
AbstractBackground: Preeclampsia (PE) is a serious pregnancy complication affecting 5-8% of pregnancies globally. It is a leading cause of maternal and neonatal morbidity and mortality. Despite its prevalence, the underlying mechanisms of PE remain unclear. This study aimed to determine the potential role of vasorin (VASN) in PE pathogenesis by investigating its levels in extracellular vesicles (EV) and its effects on vascular function. Methods & Results: We conducted unbiased proteomics on urine-derived EV from severe PE (sPE) and normotensive pregnant women (NTP), identifying differential protein abundances. Out of one hundred and twenty proteins with 1.5-fold regulation at P<0.05 between sPE and NTP, we focused on Vasorin (VASN), which is downregulated in sPE in urinary EV, in plasma EV and in the placenta and is a known regulator of vascular function. We generated EV with high VASN content from both human and murine placenta explants (Plex EV), which recapitulated disease-state-dependent effects on vascular function observed when treating murine aorta rings (MAR) or human aortic endothelial cells (HAEC) with murine or human plasma-derived EV. In normal murine pregnancy, VASN increases with gestational age (GA), and VASN is decreased in plasma EV, in placenta tissue and in Plex EV after intravenous administration of adenovirus encoding short FMS-like tyrosine kinase 1 (sFLT-1), a murine model of PE (murine-PE). VASN is decreased in plasma EV, in placenta tissue and in EV isolated from conditioned media collected from placenta explants (Plex EV) in patients with sPE as compared to NTP. Human sPE and murine-PE plasma EV and Plex EV impair migration, tube formation, and induces apoptosis in human aortic endothelial cells (HAEC) and inhibit acetylcholine-induced vasorelaxation in murine vascular rings (MAR). VASN over-expression counteracts the effects of sPE EV treatment in HAEC and MAR. RNA sequencing revealed that over-expression or knock down of VASN in HAEC results in contrasting effects on transcript levels of hundreds of genes associated with vasculogenesis, endothelial cell proliferation, migration and apoptosis. Conclusions: The data suggest that VASN, delivered to the endothelium via EV, regulates vascular function and that the loss of EV VASN may be one of the mechanistic drivers of PE.


