IL-13 and IL-17A Activate β1 Integrin through an NF-κB/Rho kinase/PIP5K1γ pathway to Enhance Force Transmission in Airway Smooth Muscle
IL-13 and IL-17A Activate β1 Integrin through an NF-κB/Rho kinase/PIP5K1γ pathway to Enhance Force Transmission in Airway Smooth Muscle
Ngo, U.; Shi, Y.; Woodruff, P.; Shokat, K.; DeGrado, W.; Jo, H.; Sheppard, D.; Sundaram, A. B.
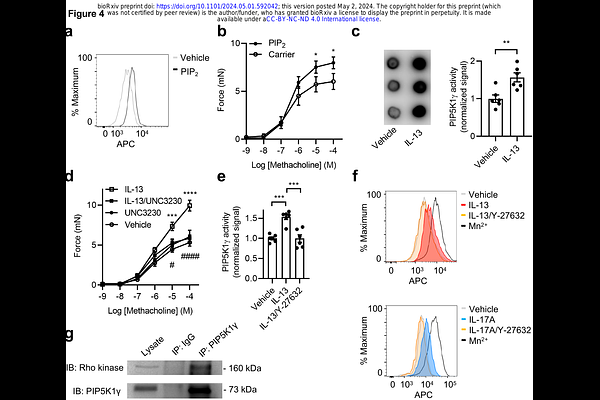
AbstractIntegrin activation resulting in enhanced adhesion to the extracellular matrix plays a key role in fundamental cellular processes. Although G-protein coupled receptor-mediated integrin activation has been extensively studied in non-adherent migratory cells such as leukocytes and platelets, much less is known about the regulation and functional impact of integrin activation in adherent stationary cells such as airway smooth muscle. Here we show that two different asthmagenic cytokines, IL-13 and IL-17A, activate type I and IL-17 cytokine receptor families respectively, to enhance adhesion of muscle to the matrix. These cytokines also induce activation of {beta}1 integrins as detected by the conformation-specific antibody HUTS-4. Moreover, HUTS-4 binding is significantly increased in the smooth muscle of patients with asthma compared to healthy controls, suggesting a disease-relevant role for aberrant integrin activation. Indeed, we find integrin activation induced by a {beta}1 activating antibody, the divalent cation manganese, or the synthetic peptide {beta}1-CHAMP, dramatically enhances force transmission in collagen gels, mouse tracheal rings, and human bronchial rings even in the absence of cytokines. We further demonstrate that cytokine-induced activation of {beta}1 integrins is regulated by a common pathway of NF-{kappa}B-mediated induction of RhoA and its effector Rho kinase, which in turn stimulates PIP5K1{gamma}-mediated synthesis of PIP2 resulting in {beta}1 integrin activation. Taken together, these data identify a previously unknown pathway by which type I and IL-17 cytokine receptor family stimulation induces functionally relevant {beta}1 integrin activation in adherent smooth muscle and help explain the exaggerated force transmission that characterizes chronic airways diseases such as asthma. Integrin activation resulting in enhanced adhesion to the extracellular matrix plays a key role in fundamental cellular processes. Although G-protein coupled receptor-mediated integrin activation has been extensively studied in non-adherent migratory cells such as leukocytes and platelets, much less is known about the regulation and functional impact of integrin activation in adherent stationary cells such as airway smooth muscle. Here we show that two different asthmagenic cytokines, IL-13 and IL-17A, activate type I and IL-17 cytokine receptor families respectively, to enhance adhesion of muscle to the matrix. These cytokines also induce activation of {beta}1 integrins as detected by the conformation-specific antibody HUTS-4. Moreover, HUTS-4 binding is significantly increased in the smooth muscle of patients with asthma compared to healthy controls, suggesting a disease-relevant role for aberrant integrin activation. Indeed, we find integrin activation induced by a {beta}1 activating antibody, the divalent cation manganese, or the synthetic peptide {beta}1-CHAMP, dramatically enhances force transmission in collagen gels, mouse tracheal rings, and human bronchial rings even in the absence of cytokines. We further demonstrate that cytokine-induced activation of {beta}1 integrins is regulated by a common pathway of NF-{kappa}B-mediated induction of RhoA and its effector Rho kinase, which in turn stimulates PIP5K1{gamma}-mediated synthesis of PIP2 resulting in {beta}1 integrin activation. Taken together, these data identify a previously unknown pathway by which type I and IL-17 cytokine receptor family stimulation induces functionally relevant {beta}1 integrin activation in adherent smooth muscle and help explain the exaggerated force transmission that characterizes chronic airways diseases such as asthma.