DFNA5-mediated pyroptosis is a driver for venetoclax and azacytidine synergy in myeloid leukemia
DFNA5-mediated pyroptosis is a driver for venetoclax and azacytidine synergy in myeloid leukemia
Mahesh, A. N.; Lai XIN-Yi, J.; Tng Jia Lin, M.; Lin, W.; Wan, J. C.; Yoon, J.; Chen, K.; Bhatt, S.
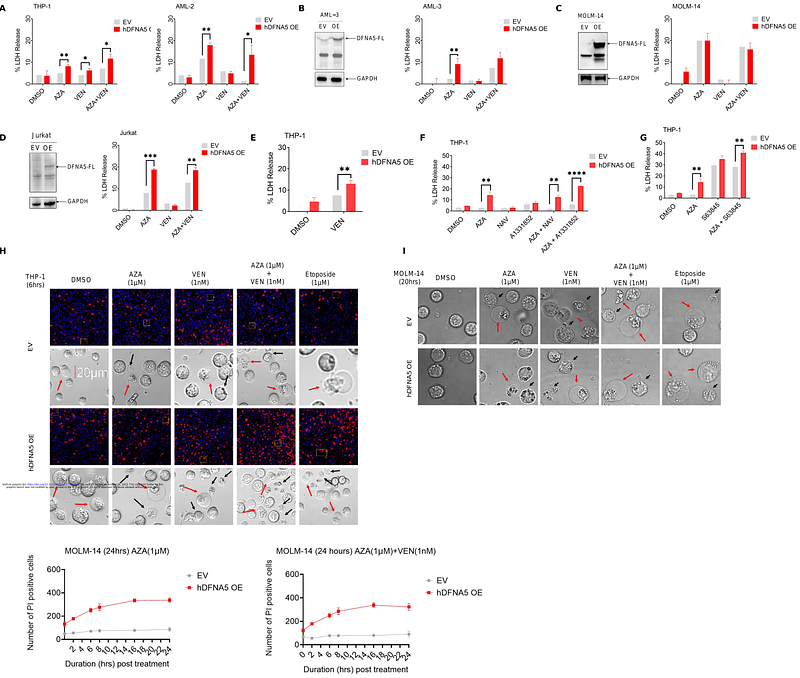
AbstractAcute myeloid leukemia (AML) remains the deadliest adult leukemia with dismal clinical outcomes. Since 2020, a combination of BCL-2 inhibitor (venetoclax, VEN) with hypomethylating agent (azacytidine/decitabine, AZA/DAC) has become a new standard of care in elderly or unfit AML patients. However, the underlying mechanism of synergy between azacytidine and venetoclax combination is not well understood. While apoptosis is regarded as the primary mode of cytotoxicity by azacytidine+venetoclax, we provide evidence for the involvement of pyroptosis in azacytidine+venetoclax-mediated cytotoxicity of AML cells. We show that long-term treatment with azacytidine caused hypomethylation and significant upregulation in DFNA5/GSDME, pore-forming protein that is otherwise silent in myeloid leukemia. We found that Azacytidine mediates N-terminal pore-forming DFNA5 cleavage, membrane rupture, and subsequent pyroptosis in DFNA5 overexpressing cells. Deletion of DFNA5 led to a significant reduction in total cell viability, where DFNA5 KO cells exclusively underwent apoptosis while DFNA5 OE cells showed increased propidium iodide uptake, a marker for membrane rupture. Overall, our study establishes DFNA5 as an important mediator of azacytidine+venetoclax-induced cell death in AML cells via both apoptotic and non-apoptotic mechanisms.