Preclinical evaluation of tissue-selective gene therapies for congenital generalised lipodystrophy
Preclinical evaluation of tissue-selective gene therapies for congenital generalised lipodystrophy
Tiwari, M.; Roumane, A.; Sommer, N.; Han, W. D.; Delibegovic, M.; Rochford, J.; Mcilroy, G. D.
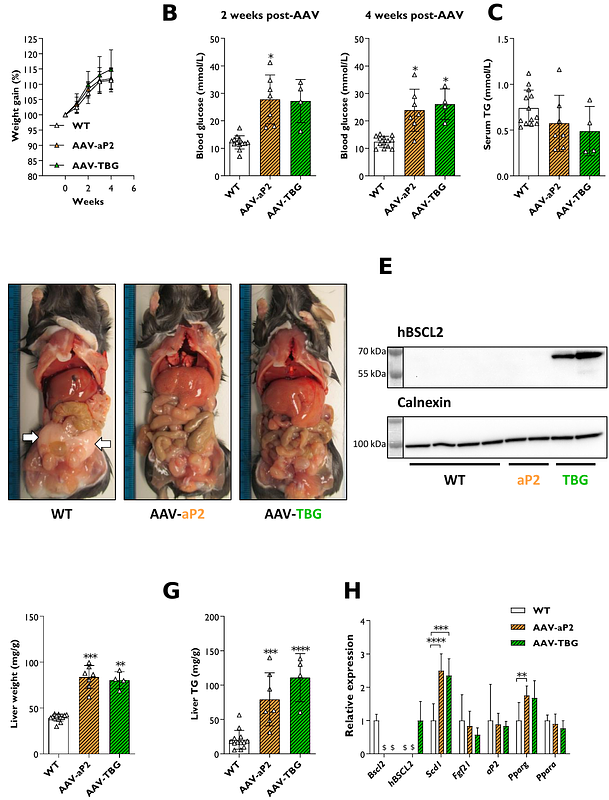
AbstractLipodystrophy is a rare disorder which can be life-threatening. Here individuals fail to develop or maintain appropriate adipose tissue stores. This typically causes severe metabolic complications, including hepatic steatosis and lipoatrophic diabetes. There is no cure for lipodystrophy, and treatment options remain very limited. Here we evaluate whether tissue-selective adeno-associated virus (AAV) vectors can provide a targeted form of gene therapy for lipodystrophy, using a preclinical lipodystrophic mouse model of Bscl2 deficiency. We designed AAV vectors containing the mini/aP2 or thyroxine-binding globulin promoter to selectively target adipose or liver respectively. The AAV-aP2 vectors also contained the liver-specific microRNA-122 target sequence, restricting hepatic transgene expression. Systemic delivery of AAV-aP2 vectors overexpressing human BSCL2 restored adipose tissue development and metabolic health in lipodystrophic mice without detectable expression in the liver. High doses (1x1012 GCs) of liver-selective vectors led to off target expression and adipose tissue development, whilst low doses (1x1010 GCs) expressed selectively and robustly in the liver but did not improve metabolic health. This reveals that adipose tissue-selective, but not liver directed, AAV-mediated gene therapy is sufficient to substantially recover metabolic health in generalised lipodystrophy. This provides an exciting potential new avenue for an effective, targeted, and thereby safer therapeutic intervention.


