Structural basis of the mechanism and inhibition of a human ceramide synthase
Structural basis of the mechanism and inhibition of a human ceramide synthase
Pascoa, T. C.; Pike, A. C. W.; Tautermann, C. S.; Chi, G.; Traub, M.; Quigley, A.; Chalk, R.; Stefanic, S.; Thamm, S.; Pautsch, A.; Carpenter, E. P.; Schnapp, G.; Sauer, D. B.
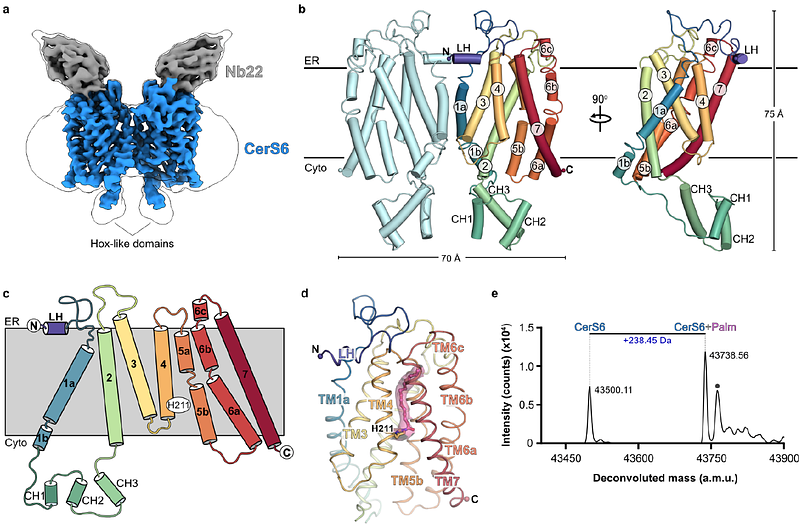
AbstractCeramides are bioactive sphingolipids that play pivotal roles in regulating cellular metabolism. Ceramides and dihydroceramides are synthesized by a family of six ceramide synthase enzymes (CerS), each with distinct specificity for the acyl-CoA substrate. Importantly, the acyl chain length plays a key role in determining the physiological function of ceramides, as well as their role in metabolic disease. Ceramide with an acyl chain length of 16 carbons (C16 ceramide) has been implicated in obesity, insulin resistance and liver disease, and the C16 ceramide-synthesizing CerS6 is regarded as an attractive drug target for obesity-associated disease. Despite their importance, the molecular mechanism underlying ceramide synthesis by CerS enzymes remains poorly understood. Here, we report cryo-electron microscopy structures of human CerS6, capturing covalent intermediate and product-bound states. These structures, together with biochemical characterization using intact protein and small molecule mass spectrometry, reveal that CerS catalysis proceeds via a ping-pong reaction mechanism involving a covalent acyl-enzyme intermediate. Notably, the product-bound structure was obtained upon reaction with the mycotoxin fumonisin B1, providing new insights into its inhibition of CerS. These results provide a framework for understanding the mechanisms of CerS function, selectivity, and inhibition, and open new directions for future drug discovery targeting the ceramide and sphingolipid pathways.