Mammalian tissue specific translation regulation; role of tRNA epitranscriptome in regulating codon optimality patterns across tissues.
Mammalian tissue specific translation regulation; role of tRNA epitranscriptome in regulating codon optimality patterns across tissues.
Ando, D.; Rashad, S.; Begley, T.; Endo, H.; Aoki, M.; Dedon, P.; Niizuma, K.
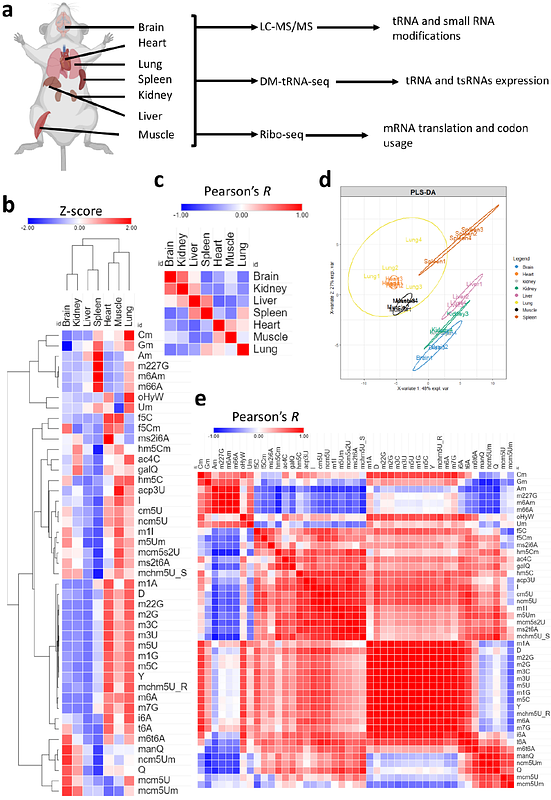
AbstractThe tRNA epitranscriptome has been recognized as an important player in mRNA translation regulation. tRNA modifications, tRNA expression, and tRNA derived small RNAs (tsRNAs) all play important roles in physiology as well as in multiple pathologies. While the decoding capacity of tRNA is generally understood, there remains gaps in our knowledge of the role of various tRNA processes in fine-tuning translation at the codon level. Specifically, how tRNAs regulates codon usage and bias at the tissue or cell levels remain unexplored. Here, we analyzed seven tissues from mice for the expression of tRNA modifications, mature tRNAs, and tsRNAs. We combined our analysis with Ribo-seq and evaluated various metrics for codon usage and optimality between tissues. Our analysis revealed distinct enrichment patterns of tRNA modifications in tissues. For example, queuosine and mcm5U modifications were most enriched in the brain. In addition, we observed lower levels of tRNAs and tsRNAs in the spleen with higher levels of snRNAs and snoRNAs compared to other tissues. Using three different metrics for codon analysis; isoacceptors frequencies, total codon frequencies, and A-site pausing, we revealed a strong A/T vs G/C ending codons bias in most tissues. The brain was the least biased tissue and was unique compared to all other tissues on multiple levels. Finally, we observed a strong concordance between the expression of queuosine modifications and the optimality of their decoded codons. Our results reveal that tRNA modifications are master regulators of translation and codon usage and optimality across tissues.