Molecular mechanism of substrate transport by human peroxisomal ABCD3
Molecular mechanism of substrate transport by human peroxisomal ABCD3
Gupta, M.; Khandelwal, N. K.; Seka, D. J.; Balasubramani, S. G.; Dickinson, M. S.; Myasnikov, A.; Echeverria, I.; Stroud, R. M.
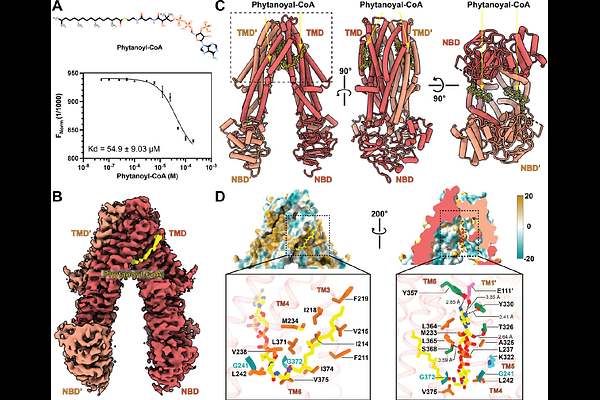
AbstractPeroxisomes are eukaryotic oxidative organelles involved in numerous metabolic functions that include fatty acid oxidation, bile acid synthesis, and detoxification of reactive oxygen species. ATP-binding cassette transporters of the D subfamily (ABCD1-3) mediate the import of CoA thioesters of fatty acids into the peroxisome. ABCD3, the most abundant of these transporters in the peroxisomal membrane, facilitates the transport of a broad spectrum of substrates including branched-chain fatty acids, very long-chain fatty acids, bile salt intermediates, and dicarboxylic acids. Mutations in ABCD3 are associated with defects in congenital bile acid synthesis and variants of Zellweger syndrome. The structural and functional details of the human ABCD3 transporter remain unclear, despite its significance. In this study, we report the cryogenic sample electron microscopy (cryo-EM) structures of full-length human ABCD3 in its apo state and bound to one of the physiological substrates (phytanoyl-CoA) at resolutions of 3.33 [A] and 3.13 [A], respectively. Our biochemical assays reveal that substrate binding induces ATPase activity in ABCD3, suggesting a substrate-dependent conformational change. Structural comparison of the apo and substrate bound states demonstrate that the substrate interaction brings nucleotide-binding domains closer, providing a mechanistic basis of substrate induced ATPase activity. These findings offer critical insights into the transport mechanism of ABCD3 and lay a structural foundation for understanding its role in peroxisomal metabolite import and related diseases.