Stepwise expansion of recombination suppression on sex chromosomes and other supergenes through lower load advantage and deleterious mutation sheltering
Stepwise expansion of recombination suppression on sex chromosomes and other supergenes through lower load advantage and deleterious mutation sheltering
Jay, P.; Veber, A.; Giraud, T.
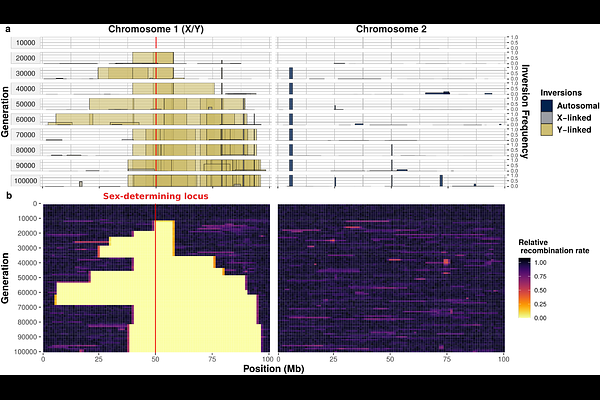
AbstractMany organisms possess sex chromosomes with non-recombining regions that have expanded progressively. Yet, the causes of this stepwise expansion remain poorly understood. Here, using mathematical modeling and stochastic simulations, we show that recombination suppression can expand simply due to the widespread presence of deleterious recessive mutations in genomes. We demonstrate that a significant proportion of new inversions are initially advantageous because they carry fewer mutations than average. However, these less-loaded inversions generally fail to fix on autosomes because, as their frequency increases, the recessive deleterious mutations they carry are more likely to occur in a homozygous state, leading to a selective disadvantage. In contrast, the permanent heterozygosity of Y-like sex chromosomes shelters sex-linked inversions from this disadvantage, facilitating their fixation and thereby the stepwise expansion of non-recombining regions. We show that this sheltering effect leads to fixation probabilities exceeding those expected under drift alone. Once recombination is suppressed, deleterious mutations accumulate on inversions, which could select for recombination restoration. However, we show that the accumulation of overlapping genomic rearrangements following recombination suppression can prevent its restoration. Our theoretical model proposes a simple and testable framework explaining evolutionary strata on sex and mating-type chromosomes, and other supergenes.