Energetic and structural control of polyspecificity in a multidrug transporter
Energetic and structural control of polyspecificity in a multidrug transporter
Miller, S. T.; Henzler-Wildman, K. A.; Raman, S.
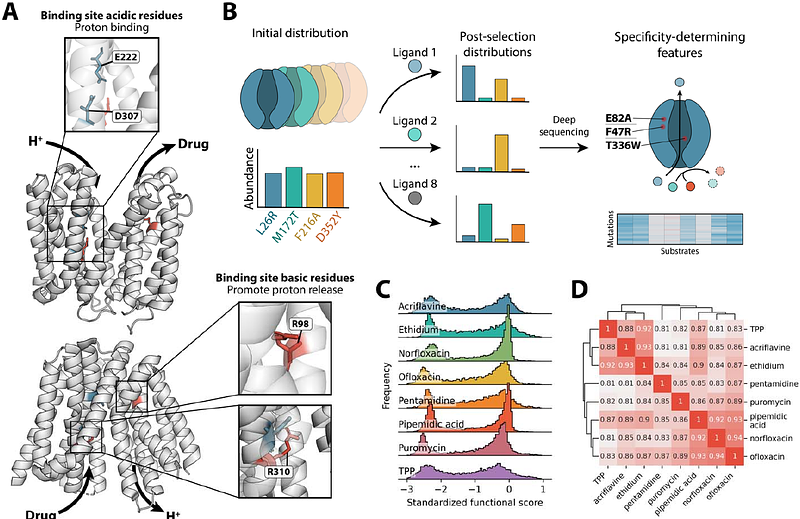
AbstractMultidrug efflux pumps are dynamic molecular machines that drive antibiotic resistance by harnessing ion gradients to export chemically diverse substrates. Despite their clinical importance, the molecular principles underlying multidrug promiscuity and energy efficiency remain poorly understood. Using multiparametric deep mutational scanning across eight substrates and two energy conditions, we deconvolute the contributions of substrate recognition, energetic coupling, and protein stability, providing an integrated, high-resolution view of multidrug transport. We find that substrate specificity arises from a distributed network of residues extending beyond the binding site, with mutations that reshape binding, coupling, conformational flexibility, and membrane interactions. Further, we apply a pH-based selection scheme to measure the effect of mutation on pH-dependent transport efficiency. By integrating these data, we reveal a fundamental relationship between efficiency and promiscuity: highly efficient variants exhibit broad substrate profiles, while inefficient variants are narrower. These findings establish a direct link between energy coupling and polyspecificity, uncovering the biochemical logic underlying multidrug transport.