Deciphering the Pathogenic Landscape of Amyloid Light-Chain Cardiomyopathy
Deciphering the Pathogenic Landscape of Amyloid Light-Chain Cardiomyopathy
Ma, Q.; Wang, Z.; Zhu, J.; Yang, D.; Liu, X.; Xu, J.; PAN, X.; Zhang, N.
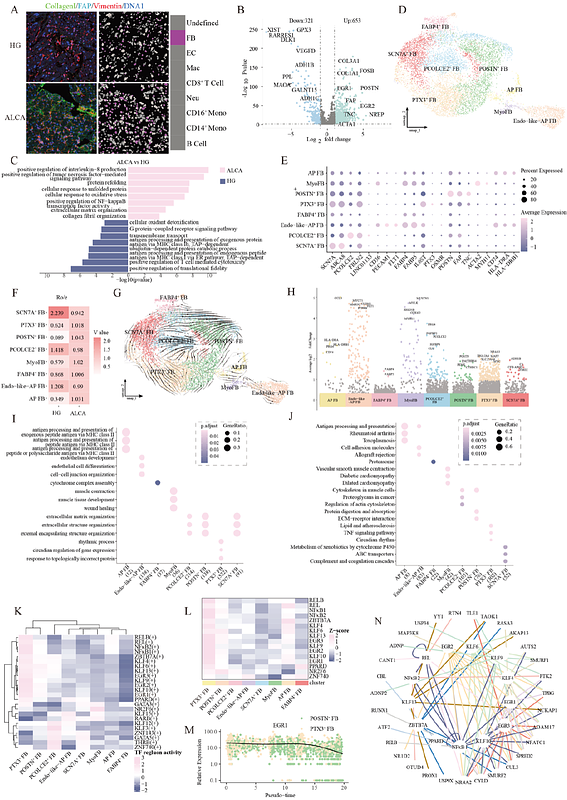
AbstractAmyloid light-chain cardiomyopathy (ALCA) is an infiltrative disorder marked by misfolded immunoglobulin light-chain deposition in the myocardium, ultimately leading to cardiac dysfunction. Despite its clinical severity, the underlying mechanisms remain poorly understood. Here, we integrated multi-omics analyses of human cardiac samples to construct a comprehensive cellular and spatial atlas of the ALCA heart. We observed a marked expansion of PTX3+ fibroblasts (FB), which undergo a distinct phenotypic transition into pro-fibrotic POSTN+ FB regulated by EGR1.Concurrently, SPP1+ macrophages (Mac) emerged as major drivers of fibrosis, interacting robustly with PTX3+ FB via APP-CD74, GAS6 MERTK, and TGF-{beta}1-TGF-{beta}1/2 pathways. Endothelial cell (EC) profiling revealed substantial vascular remodeling characterized by the emergence of specialized capillary-like immune endothelial cells expressing chemokines CXCL1, CXCL3, and CCL2, alongside depletion of functional capillary EC. Immunologically, elevated cytotoxic CD8+ T cells and reduced NK cells contributed to an imbalanced inflammatory milieu, with NF{kappa}B2 orchestrating both fibrotic and immune pathways across multiple cell types. These findings highlight the pivotal role of fibroblast-immune crosstalk, particularly the SPP1+ Mac-PTX3+ FB axis, in driving ALCA pathogenesis. Targeting these pathological cellular interactions may offer a promising therapeutic avenue to mitigate fibrosis, restore immune homeostasis, and improve cardiac function in ALCA.