Regulation of the MDM2-p53 Nexus by a Nuclear Phosphoinositide and Small Heat Shock Protein Complex
Regulation of the MDM2-p53 Nexus by a Nuclear Phosphoinositide and Small Heat Shock Protein Complex
Lee, J. H.; Chen, M.; Wen, T.; Anderson, R. A.; Cryns, V. L.
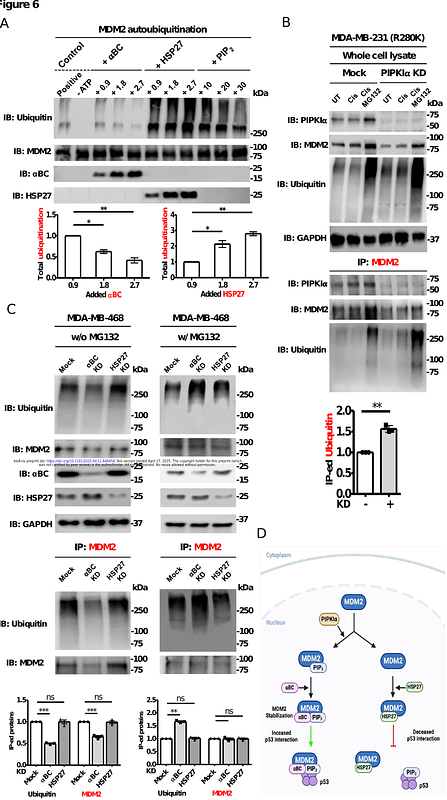
AbstractThe tumor suppressor p53 maintains genome stability in the setting of cellular stress and is frequently mutated in cancer. The stability of p53 is regulated by its interaction with the oncoprotein MDM2, a ubiquitin E3 ligase. Recently, nuclear phosphoinositides were reported to bind and stabilize p53. Here, we report that genotoxic stress induces the type I phosphatidylinositol phosphate kinase (PIPKI) and its product phosphatidylinositol 4,5-bisphosphate (PIP2) to bind and regulate the stability and function of MDM2. Following genotoxic stress, nuclear PIPKI binds to MDM2 to generate a complex of MDM2 and PIP2. PIP2 binding to MDM2 differentially regulates the recruitment of the small heat shock proteins (sHSPs) B-crystallin (BC) and HSP27 to the MDM2-PIP2 complex, acting as an on-off switch that regulates MDM2 stability, downstream targets, ubiquitination activity, and interaction with p53. Our results demonstrate an unexpected role for nuclear phosphoinositides conferring specificity to the MDM2-PIP2-sHSPs association. Notably, the differential engagement of BC and HSP27 reveals that sHSPs are not merely passive chaperones but play active, selective roles in fine-tuning MDM2 function and MDM2-p53 nexus. These findings provide a novel therapeutic strategy for targeting this pathway in cancer.