Discovery and Development of DC-174 as a Novel Oral Snakebite Treatment
Discovery and Development of DC-174 as a Novel Oral Snakebite Treatment
Chong, D. J. W.; Albulescu, L.-O.; Westhorpe, A. R.; Clare, R. H.; Marriott, A. E.; Woodley, C. M.; Gunasekar, R.; Mosallam, N.; Crittenden, E.; Stars, E.; Dawson, C. A.; Kool, J.; Wilkinson, M. C.; Leung, S. C.; Berry, N. G.; Casewell, N. R.; O.Neill, P. M.
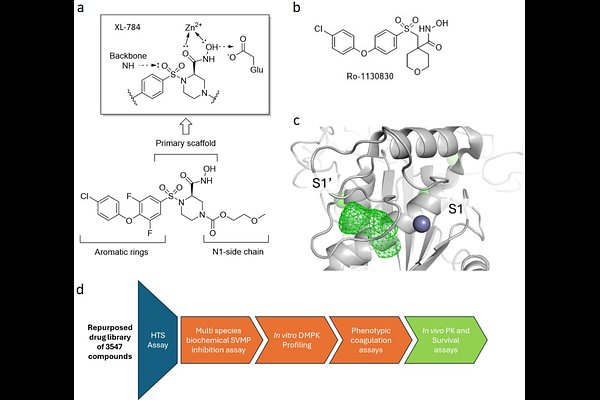
AbstractSnakebite envenoming is a neglected tropical disease that causes high mortality and morbidity. The current treatment, intravenous antivenom, comes with numerous disadvantages making new therapeutics important. Optimised small molecules offer the possibility for oral use at the onset of envenoming, and the highly pathogenic, zinc-dependent, snake venom metalloproteinase toxin family represents an attractive target for drug discovery. Through systematic chemical modification guided by molecular modelling, we describe the development of hydroxamic acid DC-174, a molecule that displays potent broad spectrum metalloproteinase inhibition and neutralises the procoagulant activities of multiple snake venoms. In oral-dosing studies, DC-174 showed preclinical efficacy in a mouse model of severe envenoming, with efficacy boosted by a pharmacokinetically-informed multiple dosing regimen. This rationally designed metalloproteinase inhibitor offers a potential paradigm shift from delayed treatment with antivenom in tertiary hospitals to a contemporary approach using oral drugs amenable for rapid use in snakebite-affected communities.