Endothelial Prolyl Hydroxylase 3 Mitigates Maladaptive Inflammation to Promote Post-Ischemic Kidney Repair
Endothelial Prolyl Hydroxylase 3 Mitigates Maladaptive Inflammation to Promote Post-Ischemic Kidney Repair
Sharma, R.; Tiwari, R.; O'Sullivan, J.; Borkowski, G. S.; An, S. Y.; Thorp, E. B.; Chandel, N. S.; Quaggin, S. E.; Kapitsinou, P. P.
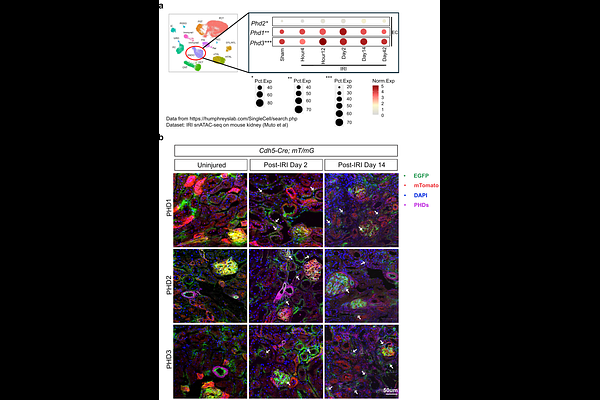
AbstractAcute kidney injury (AKI) is a major health concern and well-established risk factor for the development of chronic kidney disease (CKD). Tissue hypoxia is a prominent feature of the injured kidney that shapes the biological behavior of parenchymal and immune cells. Endothelial cells have important immunomodulatory roles, but the impact of dysregulated oxygen sensing on their responses remains poorly understood. By leveraging a combination of conditional mouse strains, single-cell analyses of mouse and human samples, and in vitro experiments, we demonstrate that the oxygen sensor PHD3 in the endothelium suppresses maladaptive inflammatory responses, thereby promoting kidney repair. Specifically, post-ischemic inactivation of endothelial PHD3, but not PHD1, leads to maladaptive kidney repair, characterized by increased fibrosis and inflammation. scRNA-seq analysis of the postischemic endothelial PHD3-haplodeficient kidney shows an endothelial IFN-{gamma} gene signature, resembling the responses seen in patients with severe AKI. By performing loss- and gain-of-function experiments in vitro, we demonstrate that PHD3 regulates IFN-{gamma} responsive pro-inflammatory signatures in a HIF-dependent manner. Consistent with this, simultaneous deletion of ARNT in endothelial PHD3-deficient mice restored kidney repair. Thus, our findings provide novel mechanistic insights into endothelial oxygen sensing and the AKI-to-CKD transition, highlighting potential therapeutic avenues for mitigating disease progression.