Extracellular Ca2+-Sensing Receptor (CaSR) Regulates Hypothalamic Function to Control Energy and Skeletal Metabolism in Mice
Extracellular Ca2+-Sensing Receptor (CaSR) Regulates Hypothalamic Function to Control Energy and Skeletal Metabolism in Mice
Park-sigal, J. J.; Norton, M.; Tu, C.-L.; Cheng, Z.; Fadahunsi, N.; Lee, H.; Kim, S.; Li, A.; Grinberg, L. T.; Bewick, G. A.; Murphy, K. G.; Chang, W. A.
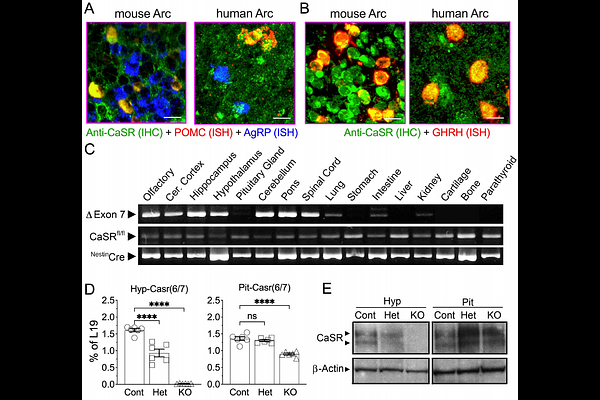
AbstractThe extracellular Calcium-sensing receptor (CaSR) regulates cellular responsiveness to physiological changes in ionized calcium (Ca2+) concentrations. The CaSR is expressed in the brain, including in hypothalamic growth hormone stimulating GHRH and anorectic POMC neurons that control growth and energy homeostasis. We embryonically deleted the Casr gene in neurons to create NeuronCaSR-/- mice to delineate the role of this receptor in regulating growth, skeletal development, and energy metabolism. NeuronCaSR-/- mice had reduced size, weight and bone mass compared to littermate controls, with a dysregulated growth hormone axis. They also showed increased adiposity and circulating leptin levels, leptin resistance, and decreased glucose tolerance, along with reduced expression of the anorectic precursor peptide POMC and secondary increases in the expression of the anorectic peptide AgRP in the hypothalamus of NeuronCaSR-/- mice. Knockdown of CaSR in adult mice specifically in the hypothalamic arcuate nucleus, where GHRH, POMC and AgRP neurons reside, also resulted in increased body weight, adiposity, leptin resistance, and glucose intolerance, and reduced bone mass. Together these data suggest that neuronal CaSR critically regulates energy and skeletal metabolism and body growth by modulating hypothalamic function, representing a new paradigm for central integration of calcaemic activities with body function.