New avenues for human blood plasma biomarker discovery via improved in-depth analysis of the low-abundant N-glycoproteome
New avenues for human blood plasma biomarker discovery via improved in-depth analysis of the low-abundant N-glycoproteome
Zuniga-Banuelos, F. J.; Hoffmann, M.; Reichl, U.; Rapp, E.
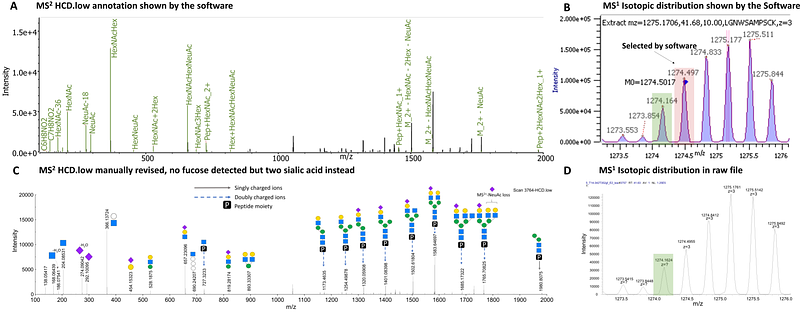
AbstractTo understand implications of protein glycosylation for clinical diagnostic and biopharmaceuticals, innovative glycoproteomic technologies are required. Recently, significant advances were made, particularly toward structure-focused N-glycoproteomic analyses. The mass spectrometric analysis of intact N-glycopeptides using stepped collision fragmentation along with glycan oxonium ion profiling now enables to reliably discriminate between different N-glycan structures. Still, there are weaknesses that current N-glycoproteomic approaches must overcome: 1) handling of incorrect identifications, 2) identification of rare and modified N-glycans, and 3) insufficient glycoproteomic coverage, especially in complex samples. To address these shortcomings, we have developed an innovative N-glycoproteomic workflow that aims at providing comprehensive site-specific and structural N-glycoproteomic data on human blood plasma glycoproteins. The workflow features protein depletion plus various fractionation strategies and the use of high-resolution mass spectrometry with stepped collision fragmentation. Furthermore, by including a decision tree procedure established for data validation, we could significantly improve the description of the N-glycan micro-heterogeneity. Our data analysis workflow allows the reliable differentiation of ambiguous N-glycan structures like antenna- versus core-fucosylation plus the modified and rare N-glycans such as sulfated and glucuronidated ones. With this workflow, we were able to advance in the analysis of human blood plasma glycoproteins to concentrations as low as 10 pg/mL. A total of 1,929 N-glycopeptides and 942 N-glycosites derived from 805 human middle- to low-abundant glycoproteins were identified. Overall, the presented workflow holds great potential to improve our understanding of protein glycosylation and to foster the discovery of blood plasma biomarkers.