SIRT4 Controls Macrophage Function and Wound Healing through Control of Protein Itaconylation in Mice
SIRT4 Controls Macrophage Function and Wound Healing through Control of Protein Itaconylation in Mice
Anderson, K. A.; deSouza, B.; Castellano-Escuder, P.; Lin, Z.; Ilkayeva, O.; Muehlbauer, M. J.; Grimsrud, P.; Hirschey, M.
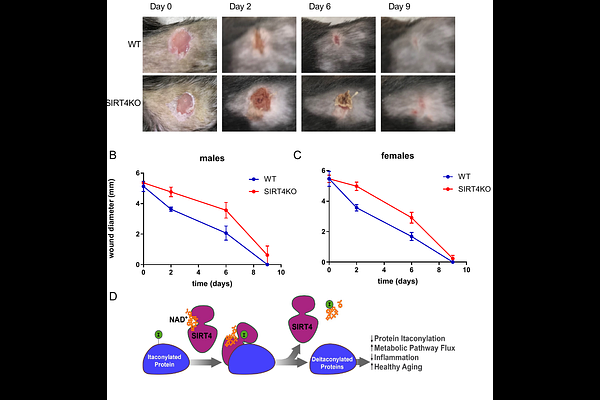
AbstractProper regulation of inflammatory responses is essential for organismal health. Dysregulation can lead to accelerated development of the diseases of aging and the aging process itself. Here, we identify a novel enzymatic activity of the mitochondrial sirtuin SIRT4 as a lysine deitaconylase that regulates macrophage inflammatory responses. Itaconate is a metabolite abundantly produced in activated macrophages. We find it forms a protein modification called lysine itaconylation. Using biochemical and proteomics approaches, we demonstrate that SIRT4 efficiently removes this modification from target proteins both in vitro and in vivo. In macrophages, elevated protein itaconylation increases upon LPS stimulation, coinciding with elevated SIRT4 expression. SIRT4-deficient macrophages exhibit significantly increased IL-1{beta} production in response to LPS stimulation. This phenotype is intrinsic to macrophages, as demonstrated by both lentiviral over-expression and acute SIRT4 knockdown models. Mechanistically, we identify key enzymes in branched-chain amino acid (BCAA) metabolism as targets of hyperitaconylation in SIRT4-deficient macrophages. The BCKDH complex component dihydrolipoamide branched chain transacylase E2 (DBT) is hyperitaconylated and has reduced BCKDH activity in SIRT4KO macrophages. Physiologically, SIRT4-deficient mice exhibit significantly delayed wound healing, demonstrating a consequence of dysregulated macrophage function. Our data reveal a novel protein modification pathway in immune cells and establish SIRT4 as a critical regulator at the intersection of metabolism and inflammation. These findings have implications for understanding immune dysregulation in aging and metabolic disease.