AT1R and Integrin β3 Synergize to Drive Aortic Dissection via Non-Canonical Wnt/β-Catenin Signaling
AT1R and Integrin β3 Synergize to Drive Aortic Dissection via Non-Canonical Wnt/β-Catenin Signaling
Jang, H.; Liu, Z.; Li, Y.; Zhong, X.; Qu, Y.; Li, S.; Dai, L.; Zhang, Y.; Wang, M.; Zhang, W.; Liu, W.; He, X.; Dong, W.; Madhusudhan, T.; Li, Z.; Wang, H.; Zeng, H.
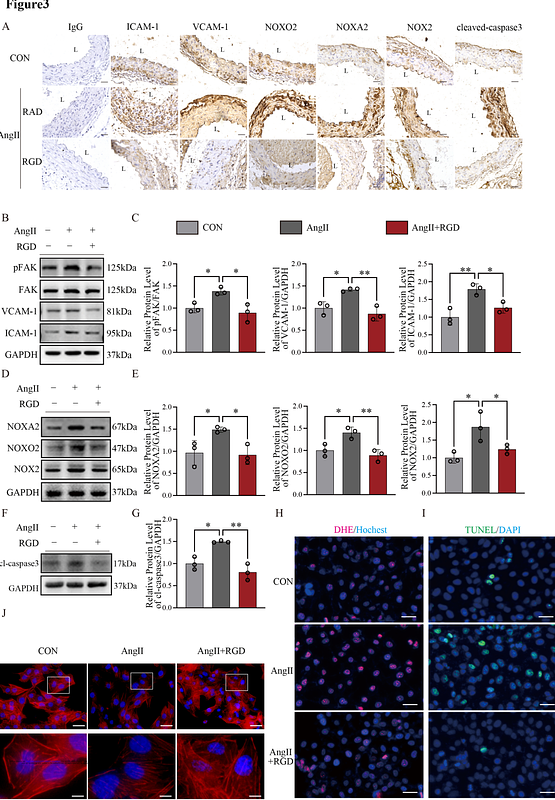
AbstractObjective: Aortic dissection (AD), a life-threatening cardiovascular emergency, continues to impose high mortality due to insufficient therapeutic options, as monotherapy targeting angiotensin II type 1 receptor (AT1R) demonstrates limited clinical efficacy. Approach and Results: Utilizing single-cell RNA sequencing, we identified integrin {beta}3 as a critical driver of AD progression, with expression levels positively correlated with disease severity. Histopathological validation in human AD specimens and a murine angiotensin II (AngII)-infusion model confirmed marked upregulation of integrin {beta}3 activation. Pharmacological blockade of integrin {beta}3 with Cyclo(-RGDfK) significantly attenuated aortic pathogenesis in vivo, reducing dissection incidence and aortic degeneration. Mechanistically, AngII-mediated AT1R activation induced formation of a receptor complex with integrin {beta}3, triggering its conformational activation. Transcriptomic profiling revealed that activated integrin {beta}3 potentiates vascular endothelial dysfunction by binding glycogen synthase kinase 3{beta} (GSK3{beta}), which stabilizes {beta}-catenin via a non-canonical Wnt signaling axis. This pathway drives endothelial barrier disruption, hallmarks of aortic wall destabilization in AD. Conclusion: Our findings unveil a previously unrecognized synergy between AT1R and integrin {beta}3, implicating aberrant Wnt/{beta}-catenin signaling as a nexus of endothelial injury in AD pathogenesis. These results advocate for a paradigm-shifting dual-therapeutic strategy concurrently targeting AT1R and integrin {beta}3 to restore vascular homeostasis, offering a mechanistically grounded approach to mitigate this lethal disease. This work bridges critical gaps in understanding AD pathophysiology and provides a transformative framework for precision therapeutics.