The penetrant chordoid glioma PRKCA mutation is an oncogenic gain-of-function kinase inactivation eliciting early onset chondrosarcoma in mice.
The penetrant chordoid glioma PRKCA mutation is an oncogenic gain-of-function kinase inactivation eliciting early onset chondrosarcoma in mice.
Calleja, v.; Henry, J. C.; Cobbaut, M.; Sewell, J.; Rizzoti, K.; Houghton, F.; Boeing, S.; Anyanwu, N.; Varsani-Brown, S.; Snoeks, T.; Suarez-Bonnet, A.; Priestnall, S. L.; McDonald, N. Q.; Cameron, A. J.; Parker, P. J.
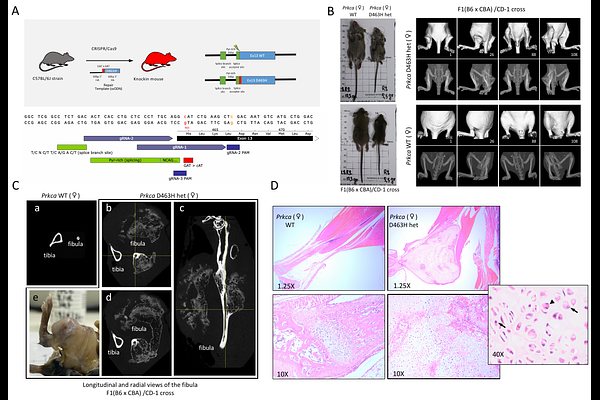
AbstractThe penetrant PRKCA D463H mutation, a biomarker and potential driver in chordoid glioma, was found to provoke the development of chondrosarcomas in heterozygous knock in mice. This mutation entirely abrogates kinase activity, but strikingly no oncogenic phenotype is observed for the related inactivating mutation D463N indicating that the lack of activity is not the driver. In cells, the D463H mutant closely mirrored PKCa WT behaviours and retained ATP binding, contrary to the related D463N mutant. Mechanistically, the PKCa D463H mutant protein was found to display quantitative alterations to the PKCa interactome, enhancing association with epigenetic regulators. This aligned with transcriptomic changes which resembled an augmented PKCa expression program, with enhanced BRD4, Myc and TGFb signatures. D463H dependent reduced sensitivity to the BET inhibitors JQ1 and AZD5153 indicates the functional importance of these pathways. The data show that D463H is a dominant gain-of-function oncogenic mutant, operating through a non-catalytic allosteric mechanism.