Intestinal fructose metabolism/GLP-1/β-cell axis counteracts hyperglycemia after short-term fructose ingestion
Intestinal fructose metabolism/GLP-1/β-cell axis counteracts hyperglycemia after short-term fructose ingestion
Murao, N.; Seino, Y.; Morikawa, R.; Hidaka, S.; Haraguchi, T.; Tomatsu, E.; Habara, M.; Ohno, T.; Yokoi, N.; Harada, N.; Hayashi, Y.; Yamada, Y.; Suzuki, A.
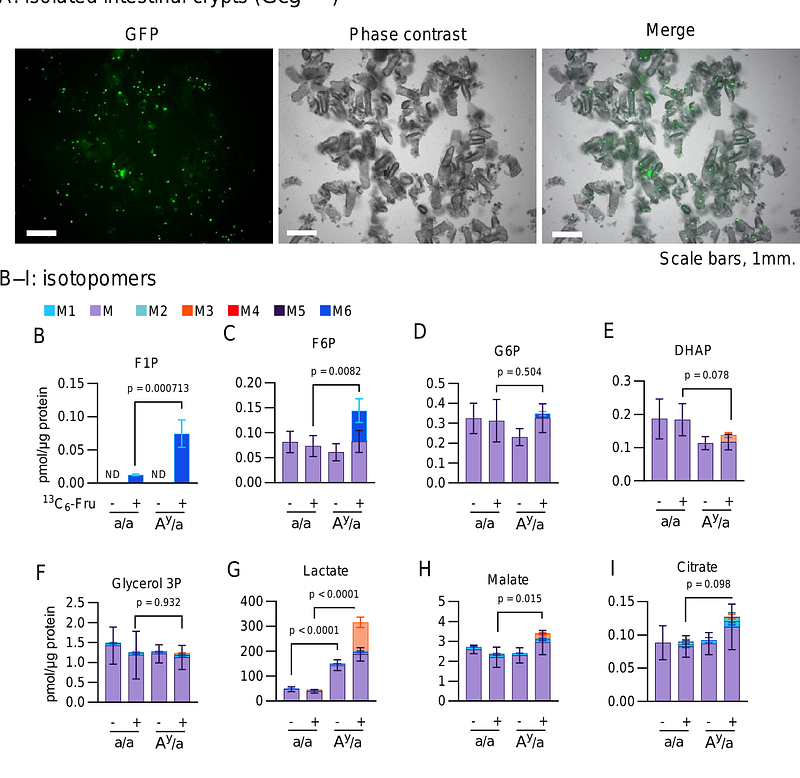
AbstractObjective The endocrine mechanisms linking fructose ingestion to glycemia remain unclear. This study revisited the mechanisms of fructose-induced incretin and insulin secretion to contextualize their roles in glycemic regulation following short-term fructose administration. Methods Mice were administered 20% fructose water ad libitum for 24 h. Plasma insulin and GLP-1 levels were compared with those of the water-administered controls. The mouse models included lean mice (C57BL/6J and NSY.B6-a/a), obese diabetic mice (NSY.B6-Ay/a), proglucagon-deficient mice (Gcg-/-), glucose-dependent insulinotropic polypeptide (GIP) receptor-deficient mice (Gipr-/-), and ATP-sensitive potassium channel (KATP channel)-deficient mice (Kcnj11-/-). The involvement of intracellular fructose metabolism in GLP-1 secretion was investigated using GLUTag cells (a mouse L-cell line) and freshly isolated intestinal crypts. Results Fructose ingestion increased insulin, GLP-1, and GIP levels in lean mice. This insulin response was maintained in Gipr-/- mice but was eliminated in Gcg-/- mice or upon administration of the GLP-1 receptor antagonist exendin [9-39], indicating the requirement of GLP-1. Notably, multiple mouse strains deficient in fructose-induced insulin responses exhibit hyperglycemia following fructose ingestion, suggesting that insulin responses are essential for suppressing glycemia. Characterization of Kcnj11-/- mice and GLUTag cells corroborated the hypothesis that intestinal fructose metabolism regulates GLP-1 secretion through the ATP/ADP ratio and KATP channels. Metabolic tracing of the isolated intestinal crypts further suggested that fructolysis activity is coupled to the ATP/ADP ratio in the L-cell-resident niche. Conclusion Fructose ingestion stimulates GLP-1 secretion through intestinal fructose metabolism, which is required for the potentiation of insulin secretion. This pathway constitutes a counter-regulatory response to fructose-induced hyperglycemia.