Validation of a high-throughput screening assay for the characterization of cryoprotective agent toxicity
Validation of a high-throughput screening assay for the characterization of cryoprotective agent toxicity
Jaskiewicz, J. J.; Callahan-Muller, A.; Gaby-Biegel, N.; Glover, Z.; Sandlin, R.
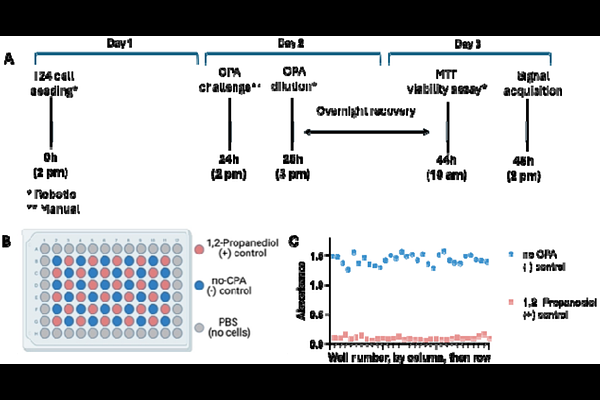
AbstractCryoprotective agent (CPA) toxicity is a major limitation in cryobiology. The discovery of new CPAs and formulation of low toxicity CPA cocktails can be streamlined by use of high-throughput screening (HTS) assays. HTS allows for rapid examination of a large chemical space, but assays should be rigorously validated to ensure the production of high-quality datasets. Here, we report the development of a CPA toxicity assay, validation of the assay for HTS using prevailing statistical metrics, and completion of a pilot screen to assess assay performance against various CPA cocktail formulations. The resulting assay, which consists of T24 cell monolayers in 96-well microtiter plates, was found to exhibit favorable performance including a Z-factor of 0.75, with intra-assay coefficient of variation and drift of <20%, which indicates that the assay is suitable for HTS implementation. A pilot screen was then completed using 587 unique CPA cocktails with concentrations ranging from 3.5-8 M. Each cocktail was tested with multiple replicates to characterize plate-to-plate and day-to-day variability of the assay (N=2-9, 2,352 total experiments). Of the 56 plates examined in the pilot screen, 53 exhibited favorable performance based on assessment of the on-plate controls (95% success rate). CPA cocktails tested at a concentration of 5 M were found to be highly informative, where cell survival ranged from 2.8-87.3%, with a favorable hit rate of 1.7%, defined here as cell viability [≥]80%.