The BRD4-nucleosome interaction is enhanced modestly and non-selectively by histone acetylation
The BRD4-nucleosome interaction is enhanced modestly and non-selectively by histone acetylation
Lambrechts, L. S.; Reid, X. J.; Kambanis, L.; Luong, C.; Kobakhidze, E.; Daners, A.; Zhong, Y.; Patel, K.; Franck, C. K.; Sani, H. M.; Taylor, C.; Low, J. K.; Payne, R. J.; Mackay, J. P.
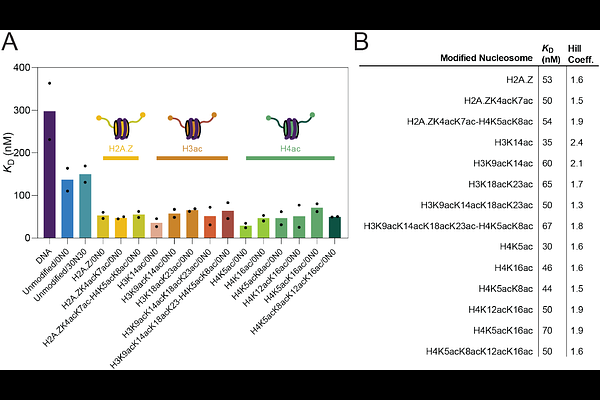
AbstractBRD4 regulates gene transcription in complex eukaryotes, in part through the binding of its tandem bromodomains to acetylated lysine residues found in histones and transcription factors. Despite pharmacological inhibition of these domains showing promise in preclinical studies, clinical trial data have been less encouraging so far. A stronger understanding of BRD4 biochemistry could provide a route to better outcomes. To advance on prior work, which has focused almost entirely on the binding of isolated bromodomains and acetylated peptides, we have sought the preferred nucleosomal binding partner of full-length BRD4. We demonstrate that BRD4 binds with sub-micromolar affinity to both unmodified nucleosomes and to DNA alone. In strong contrast to BRD4-peptide interactions, we also find that the affinity of BRD4 for nucleosomes is increased only 2-4-fold by histone acetylation and that this affinity has little dependence on the acetylation pattern. Despite this modest effect of acetylation, binding of BRD4 to acetyllysine in the nucleosome was more resistant to perturbation by mutation or small-molecule inhibition than BRD4-peptide interactions. Our work helps bridge the gap between cellular and prior in vitro work and provides clues to explain the in vivo chromatin occupancy profile of BRD4 and how it changes upon therapeutic inhibition.