MORC2 phosphorylation fine tunes its DNA compaction activity
MORC2 phosphorylation fine tunes its DNA compaction activity
Tan, W.; Park, J. V.; Venugopal, H.; Lou, J. Q.; Dias, P. S.; Baldoni, P.; Dite, T.; Moon, K.-W.; Keenan, C. R.; Gurzau, A. D.; Leis, A.; Yousef, J.; Vaibhav, V.; Dagley, L.; Ang, C.-S.; Corso, L.; Davidovich, C.; Vervoort, S.; Smyth, G. K.; Blewitt, M. E.; Allan, R. S.; Hinde, E.; D'Arcy, S.; Ryu, J.-K.; Shakeel, S.
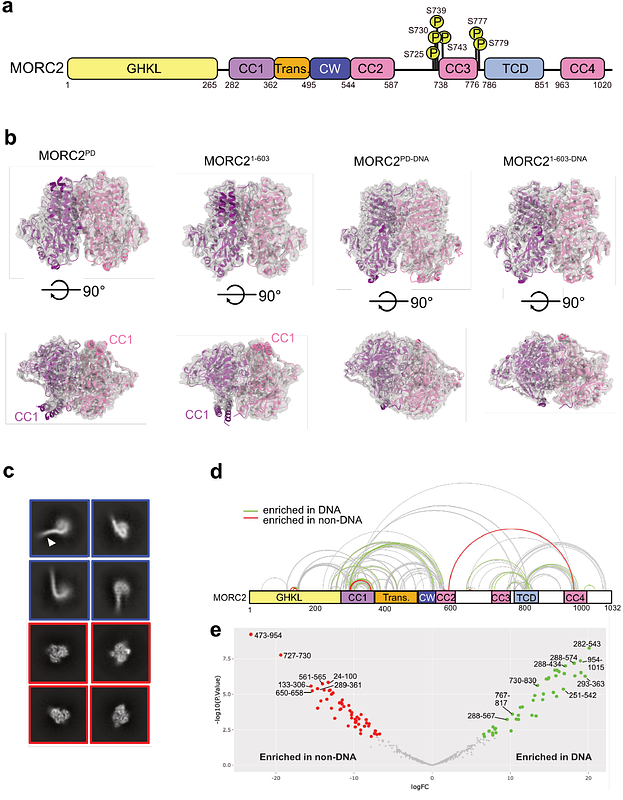
AbstractVariants in the poorly characterised oncoprotein, MORC2, a chromatin remodelling ATPase, lead to defects in epigenetic regulation and DNA damage response. The C-terminal domain (CTD) of MORC2, frequently phosphorylated in DNA damage, promotes cancer progression, but its role in chromatin remodelling remains unclear. Here, we report a molecular characterisation of full-length, phosphorylated MORC2, demonstrating its preference for binding open chromatin and functioning as a DNA sliding clamp. We identified a phosphate interacting motif within the CTD that dictates ATP hydrolysis rate and cooperative DNA binding. The DNA binding impacts several structural domains within the ATPase region. We provide the first visual proof that MORC2 induces chromatin remodelling through ATP hydrolysis-dependent DNA compaction, regulated by its phosphorylation state. These findings highlight phosphorylation of MORC2 CTD as a key modulator of chromatin remodelling, presenting it as a potential therapeutic target.


