Ethosuximide: subunit- and Gβγ-dependent blocker and reporter of allosteric changes in GIRK channels
Ethosuximide: subunit- and Gβγ-dependent blocker and reporter of allosteric changes in GIRK channels
Shalomov, B.; Friesacher, T.; Yakubovich, D.; Combista, J. C.; Reddy, H. P.; Dabbah, S.; Bernsteiner, H.; Zangerl-Plessl, E.-M.; Stary-Weinzinger, A.; Dascal, N.
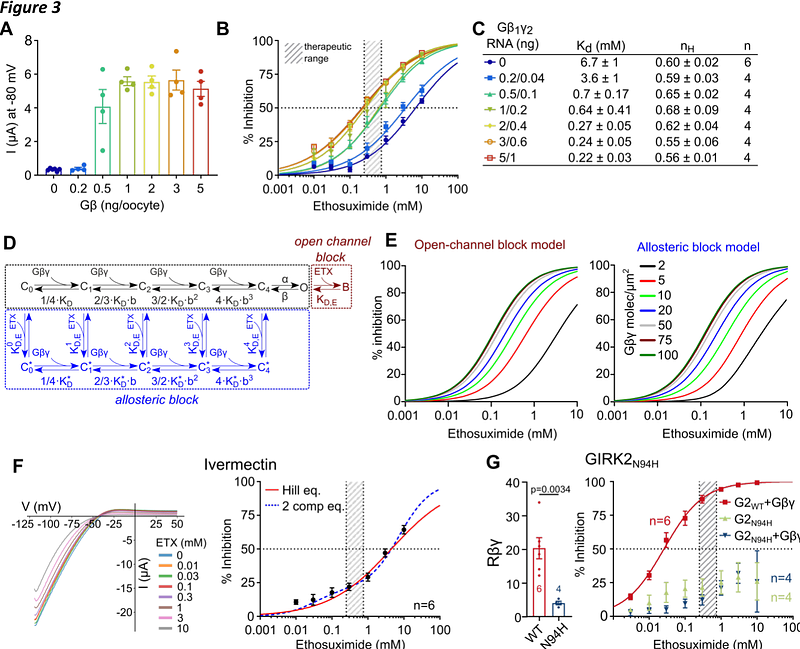
AbstractThe antiepileptic drug ethosuximide (ETX) suppresses epileptiform activity in a mouse model of GNB1 syndrome, caused by mutations in G{beta}1 protein, likely through the inhibition of G-protein gated K+ (GIRK) channels. Here we show that ETX is a subunit-selective, allosteric blocker of GIRKs. The potency of ETX block is increased by the G protein subunit dimer G{beta}{gamma}, the physiological activator of GIRKs. Molecular dynamics (MD) simulations and mutagenesis locate the ETX binding site in GIRK2 to a region associated with phosphatidylinositol-4,5-bisphosphate (PIP2) regulation, and suggest that ETX acts by closing the HBC gate and altering channel\'s interaction with PIP2. The apparent affinity of ETX block is highly sensitive to changes in channel gating caused by mutations in G{beta}1 or GIRK subunits. Our findings pose GIRK as a potential therapeutic target for ETX, and ETX as a potent allosteric GIRK blocker and a tool for probing gating-related conformational changes in GIRK.


