Preclinical study of the human recombinant Erythropoietin (GBPD002) compared with Eprex
Preclinical study of the human recombinant Erythropoietin (GBPD002) compared with Eprex
Nag, K.; Mohiuddin, M.; Khan, M. M. R.; Kumar, S.; Sarker, M. E. H.; Roy, R.; Biswas, B. K.; Barman, U.; Sultana, N.; Bachar, S. C.; Haq, S. R.
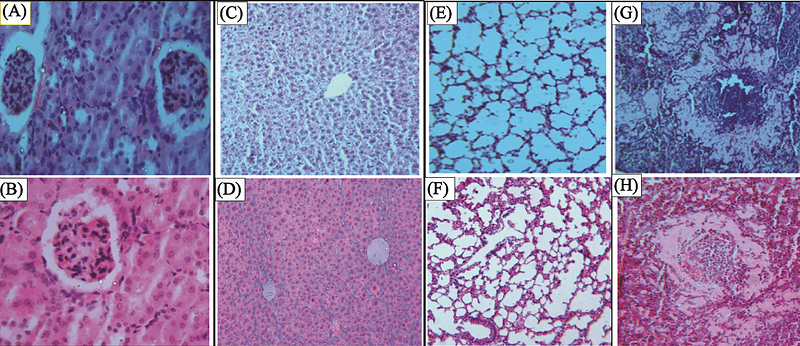
AbstractErythropoietin (EPO) is a glycoprotein that an essential growth factor for erythroid progenitors at the bone marrow, which appears in blood as a response to hypoxia. It is produced mainly by the kidneys; and its biosynthesis and release are stimulated by the reduction of tissue oxygenation and/or the reduction of the mass of erythrocytes. Here, we report the results of the preclinical evaluation of the safety of GBPD002 a recombinant human erythropoietin (rhEPO) developed by the Globe Biotech Limited, Bangladesh, through a comparative study of acute toxicity with Eprex, a commercial homologue from Janssen, UK. The product was administered subcutaneously into Wistar rats, at 500 lU/kg of therapeutic dose (TD) and 3 times of TD for the single dose toxicity study on 14 consecutive days and 125 UL/kg, 250 UL/kg, 500 UL/kg, 750 UL/kg, 1000 UL/kg, 1250 UL/kg and 1500 UL/kg of GBPD002 and Eprex on 7 consecutive days respectively for the repeated dose toxicity study. Hematological and biochemical parameters were measured for all test subjects before first dose injection and the day after last dose injection of the both studies. Necropsy and histopathology of representative subjects from each group were also observed to find any pathological significance like degeneration or cellular necrosis in internal organs such as kidney, liver, lung and spleen of any rat under experiment. Both GBPD002 and Eprex comparative toxicology studies revealed similar pharmacologically driven mechanisms of toxicity, which is statistically insignificant (p >0.05). Though hematology parameter values stayed within the normal range during the assay period but the high count of hemoglobin and high hematocrit (P<0.05), together with the decrease in white blood cell, confirm the therapeutic effect of Erythropoietin in both studies. Moreover, in both studies, the initial and final values of aspartate aminotransferase, alanine aminotransferase and blood urea nitrogen were also found similar (p >0.05) for both GBPD002 and Eprex in the study. The study clearly established that the toxicological profile of GBPD002 and Eprex, administered subcutaneously, were similar and related to the known pharmacology of erythropoietin alfa; hereby, demonstrating the proof of totality and no residual uncertainty between GBPD002 and Eprex. Therefore, GBPD002 and Eprex shall be administered interchangeably in relevant indications.


