A new heme enzyme family forms hydrazine groups in diverse biosynthetic pathways
A new heme enzyme family forms hydrazine groups in diverse biosynthetic pathways
Kenney, G. E.; Wang, K.-K. A.; Ng, T.; van der Donk, W.; Balskus, E. P.
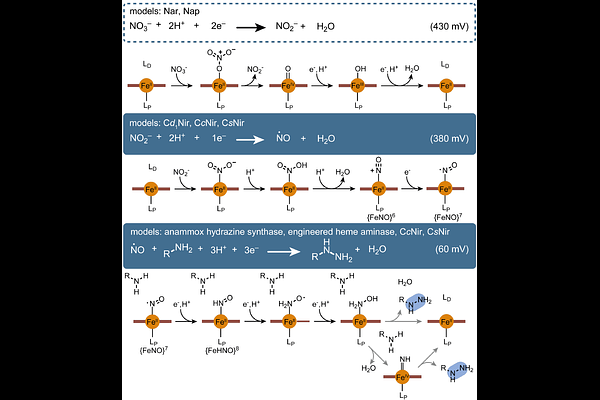
AbstractNitrogen-nitrogen (N-N) bond formation is an inherently challenging chemical process that plays a key role in the global nitrogen cycle. An array of microbial metalloenzyme complexes has evolved to shuttle nitrogen between biologically accessible reduced or oxidized states and its inert form as dinitrogen (N2) gas. More recently, N-N bond formation has been observed in a more specialized context, natural product biosynthesis. Here, we report the discovery of a unique metalloenzyme complex that forms hydrazine functional groups in the biosynthetic pathways of structurally diverse natural products. This heterodimeric system consists of a heme enzyme from a previously unidentified family and a partner ferredoxin. Together, these enzymes effect the unprecedented four-electron reduction of nitrite (NO2-) to form a hydrazine functional group on a substrate amino acid in an oxygen-independent reaction that resembles primary microbial nitrogen metabolism. These enzymes are unexpectedly widespread among bacteria and are present in diverse genomic contexts, including cryptic biosynthetic gene clusters, highlighting the importance of this previously uncharacterized protein family.