H2B.W2, a Spermatocytes-specific Histone Variant, disrupts nucleosome stability and reduces chromatin compaction
H2B.W2, a Spermatocytes-specific Histone Variant, disrupts nucleosome stability and reduces chromatin compaction
NGUYEN, T. T.; Ding, D.; Bai, X.; Pang, M. Y. H.; Mingxi, D.; Liu, Y.; Jin, T.; Zhichun, X.; Zhang, Y.; Zhai, Y.; Yan, Y.; Ishibashi, T.
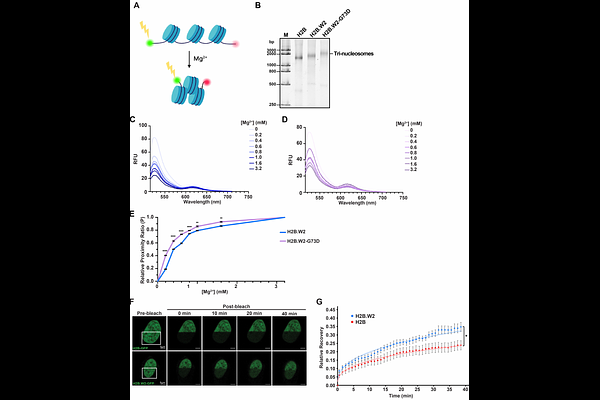
AbstractSpermatogenesis, a tightly regulated process, necessitates precise chromatin remodeling which involves the incorporation of testis-specific histone variants. While several such variants have been characterized, the role of H2B.W2, a member of the H2BW family, remains largely elusive. Here, we showed that H2B.W2 expression occurs mainly in spermatocytes, slightly later than its paralog H2B.W1. Cryo-electron microscopy (cryo-EM) analysis of H2B.W2-containing nucleosomes reveals a more relaxed conformation compared to canonical nucleosomes caused by weakened interactions between the outer DNA turn and the histone core. We pinpointed the N-terminal tail and 2 helix of H2B.W2, specifically residues D85 and Q101, as critical for nucleosome destabilization. Furthermore, we identify G73 within the L1 loop as a key residue involved in disrupting higher-order chromatin structure. Our findings suggest that H2B.W2-mediated nucleosome and chromatin destabilization may play a role in regulating gene expression during spermatogenesis, with potential implications for sperm development and function.