Endothelial c-IAP2 Loss Amplifies P2X7 Receptor-Driven Inflammation and Worsens Infection-Associated Pulmonary Hypertension
Endothelial c-IAP2 Loss Amplifies P2X7 Receptor-Driven Inflammation and Worsens Infection-Associated Pulmonary Hypertension
Villarreal, E. S.; Marinho, Y.; Loya, O.; Aboagye, S. Y.; Williams, D. L.; Sun, J.; Erzurum, S.; de Jesus Perez, V.; Oliveira, S. D.
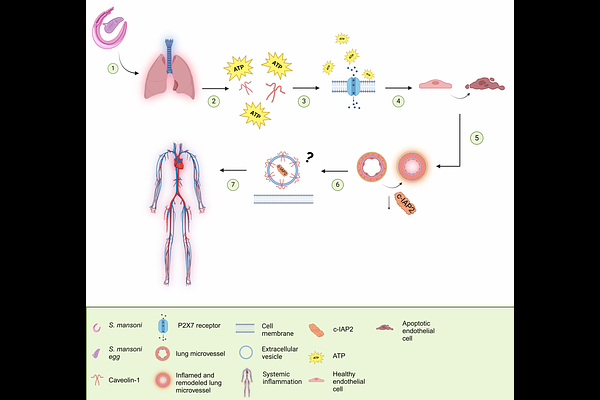
AbstractSchistosomiasis-associated Pulmonary Hypertension (Sch-PH) is the most common form of group I PH worldwide. Recently, data revealed that the preclinical animal model of Sch-PH exhibited gut and lung microbiome dysbiosis linked to significant endothelial dysfunction and microvascular apoptosis, but the role of pro/anti-apoptosis sensors, such as the inhibitor of apoptosis protein 2 (c-IAP2) and purinergic receptor P2X7 (P2X7R), remained unclear. Using a novel Cdh5cre-ERT2;cIAP1-/-;cIAP2fl/fl animal model, this study investigated the contribution of endothelial c-IAP2 in this process, revealing P2X7R overexpression as a putative target in the onset of Sch-PH. Pharmacologically, inhibition of P2X7R function confirmed its role in promoting lung endothelial death and disease progression. Moreover, data suggest that microbiome-associated metabolic alterations in Sch-PH seem linked to microvascular endothelial apoptosis driven by ATP/P2X7R overactivation and suppressed c-IAP2 expression. Indeed, genetic ablation of endothelial c-IAP2 expression was sufficient to induce PH-like features in mice, with echocardiography indicating a higher pulmonary acceleration time (PAT), PAT/pulmonary ejection time (PET), and right ventricular free wall thickness after IP/IV-Egg challenge compared to controls. These findings suggest a significant contribution of lung endothelial P2X7R activation and c-IAP2 suppression to Sch-PH pathology, highlighting them as promising novel therapeutic targets for this life-threatening illness.